Fuel cell cathode catalyst with high stability suitable for dynamic conditions
A high-stability technology for fuel cell cathodes, applied in battery electrodes, catalyst carriers, physical/chemical process catalysts, etc., can solve the problems of low catalyst stability and achieve suitable for large-scale production, high catalyst stability, and conditions easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Mix the raw material MWNTs (diameter: 8nm) with concentrated nitric acid (65-68%) at a ratio of 1g:100mL, treat with ultrasonic waves for 15min, then reflux at 120°C for 4h, cool to room temperature, filter, and wash the solid with distilled water several times To neutral, dry overnight at 80°C in a vacuum oven.
[0037] The carbon nanotube-supported platinum catalyst was prepared by intermittent microwave heating method, specifically: firstly, the treated MWNTs and ethylene glycol were mixed and ultrasonically stirred until uniform, and then an appropriate amount of H 2 PtCl 6The solution was ultrasonically stirred, and finally the pH value was adjusted to pH>12 with NaOH in ethylene glycol solution. Then heat it in a household microwave oven, cool it to room temperature, add dilute hydrochloric acid to adjust the pH value to pH<2, and after centrifugal washing to no chloride ion, vacuum dry it in a vacuum oven at 80°C overnight. The loading of Pt was 20%.
[0038] ...
Embodiment 2
[0040] Mix the raw material MWNTs (diameter: 10-20nm) with concentrated nitric acid (65-68%) at a ratio of 1g:100mL, treat with ultrasonic waves for 15min, then reflux at 120°C for 4h, cool to room temperature, filter, and wash the solid with distilled water Multiple times to neutral, dry overnight at 80°C in a vacuum oven.
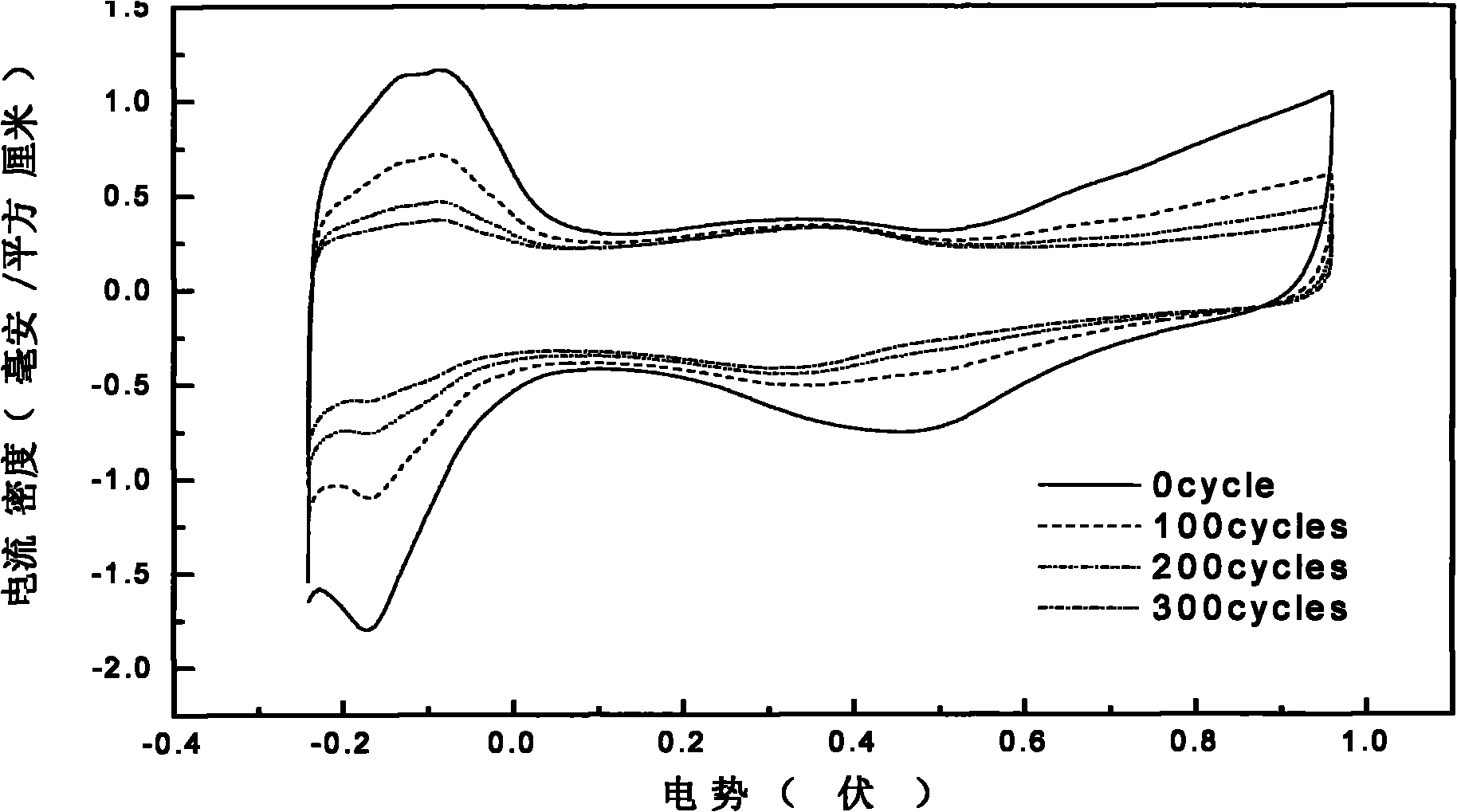
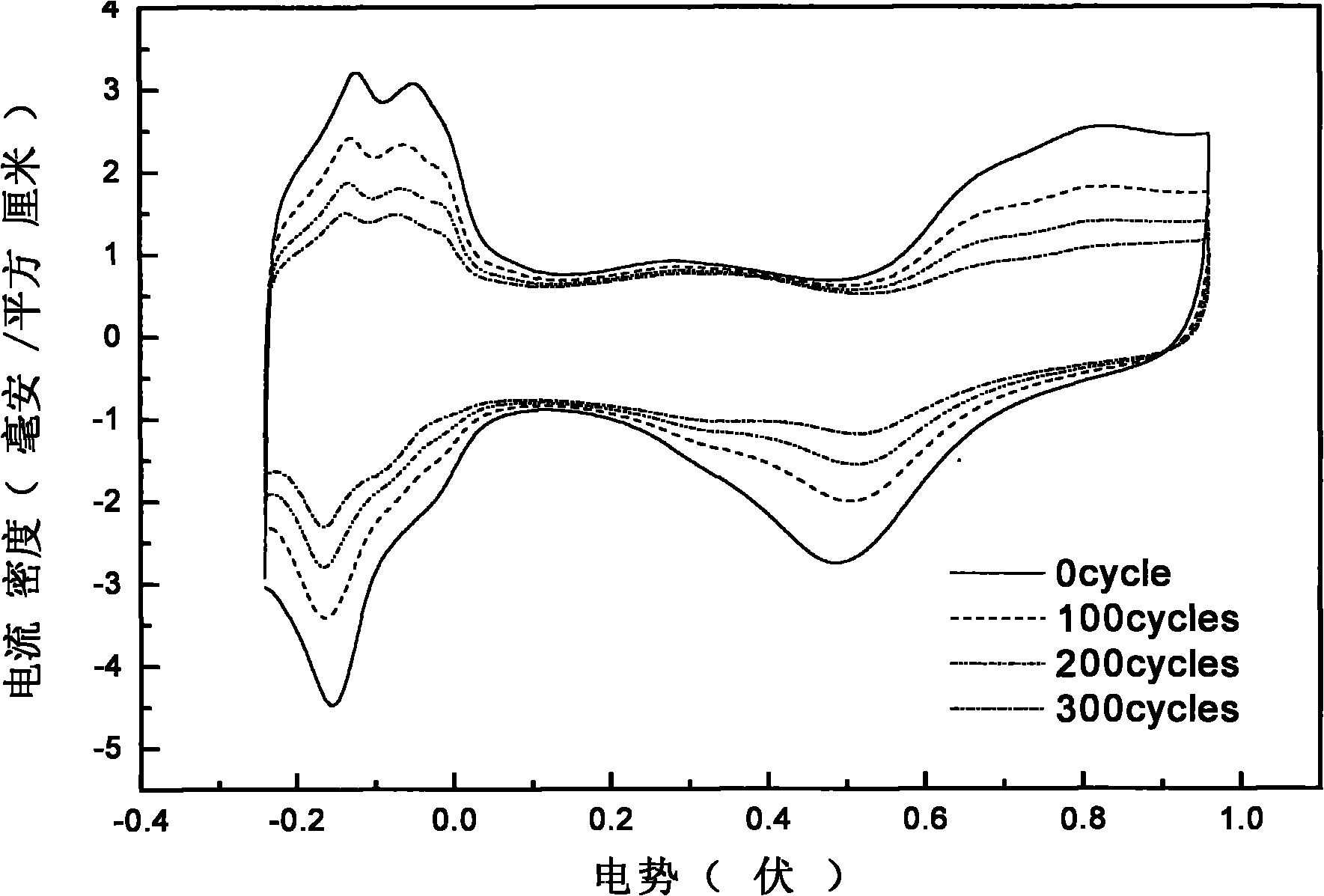
[0041] The carbon nanotube-supported platinum catalyst was prepared by intermittent microwave heating method, specifically: firstly, the treated MWNTs and ethylene glycol were mixed and ultrasonically stirred until uniform, and then an appropriate amount of H 2 PtCl 6 The solution was ultrasonically stirred, and finally the pH value was adjusted to pH>12 with NaOH in ethylene glycol solution. Then heat it in a household microwave oven, cool it to room temperature, add dilute hydrochloric acid to adjust the pH value to pH figure 2 , 5 It is the CV and ORR diagrams before and after 300 scans of the catalyst prepared from the treated carbon nanotubes in th...
Embodiment 3
[0043] Mix the raw material MWNTs (diameter: 30-50nm) with concentrated nitric acid (65-68%) at a ratio of 1g:100mL, treat with ultrasonic waves for 15min, then reflux at 120°C for 4h, cool to room temperature, filter, and wash the solid with distilled water Multiple times to neutral, dry overnight at 80°C in a vacuum oven.
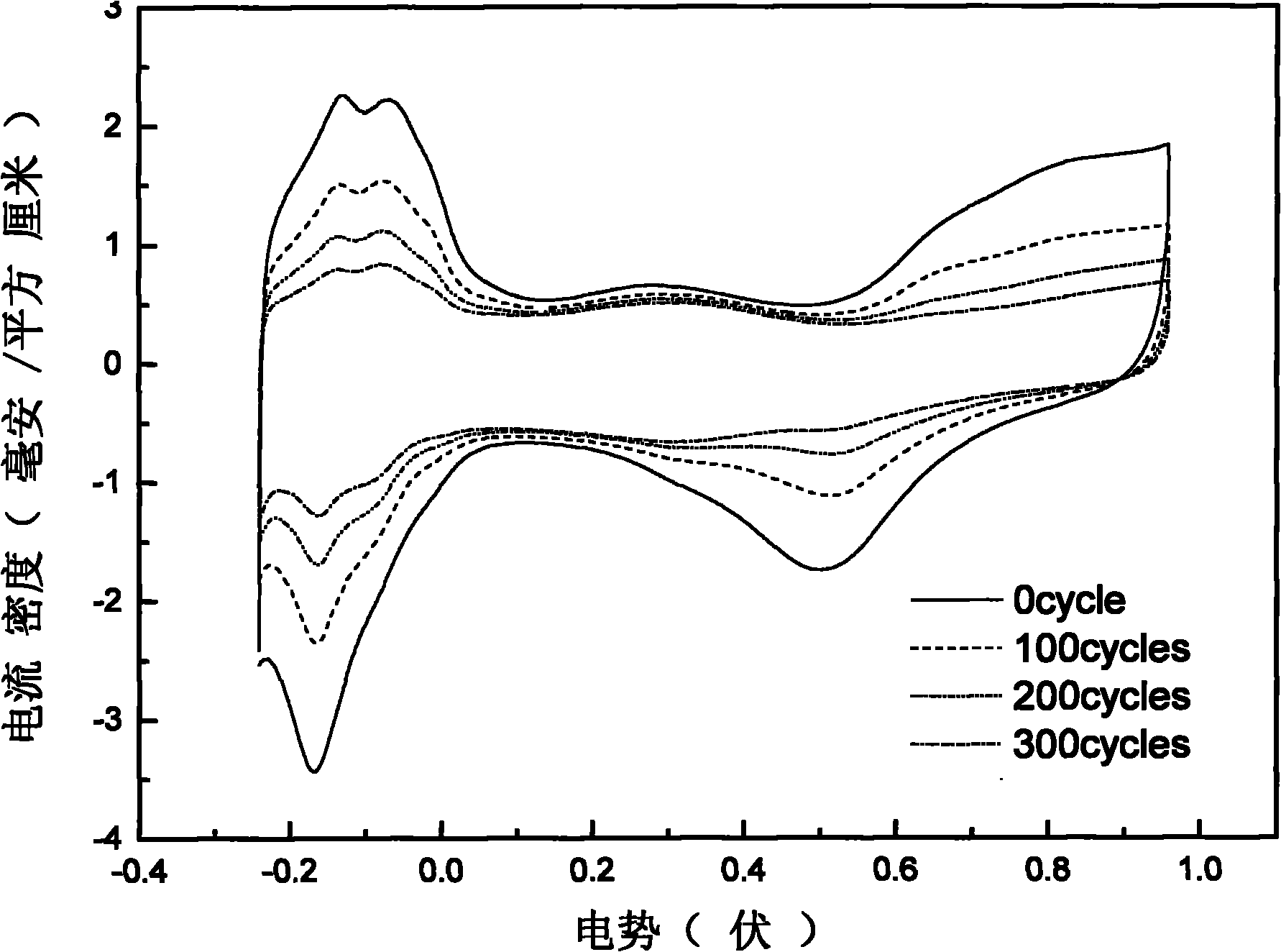
[0044] The carbon nanotube-supported platinum catalyst was prepared by intermittent microwave heating method, specifically: firstly, the treated MWNTs and ethylene glycol were mixed and ultrasonically stirred until uniform, and then an appropriate amount of H 2 PtCl 6 The solution was ultrasonically stirred, and finally the pH value was adjusted to pH>12 with NaOH in ethylene glycol solution. Then heat it in a household microwave oven, cool it to room temperature, add dilute hydrochloric acid to adjust the pH value to pH image 3 , 6 It is the CV and ORR diagrams before and after 300 scans of the catalyst prepared from the treated carbon nanotubes in thi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com