Fusion protein of antibody targeted complement regulatory factor for treating myasthenia gravis
A fusion protein and complement technology, applied in the field of medicine and biology, can solve problems such as the reduction of DAF gene expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
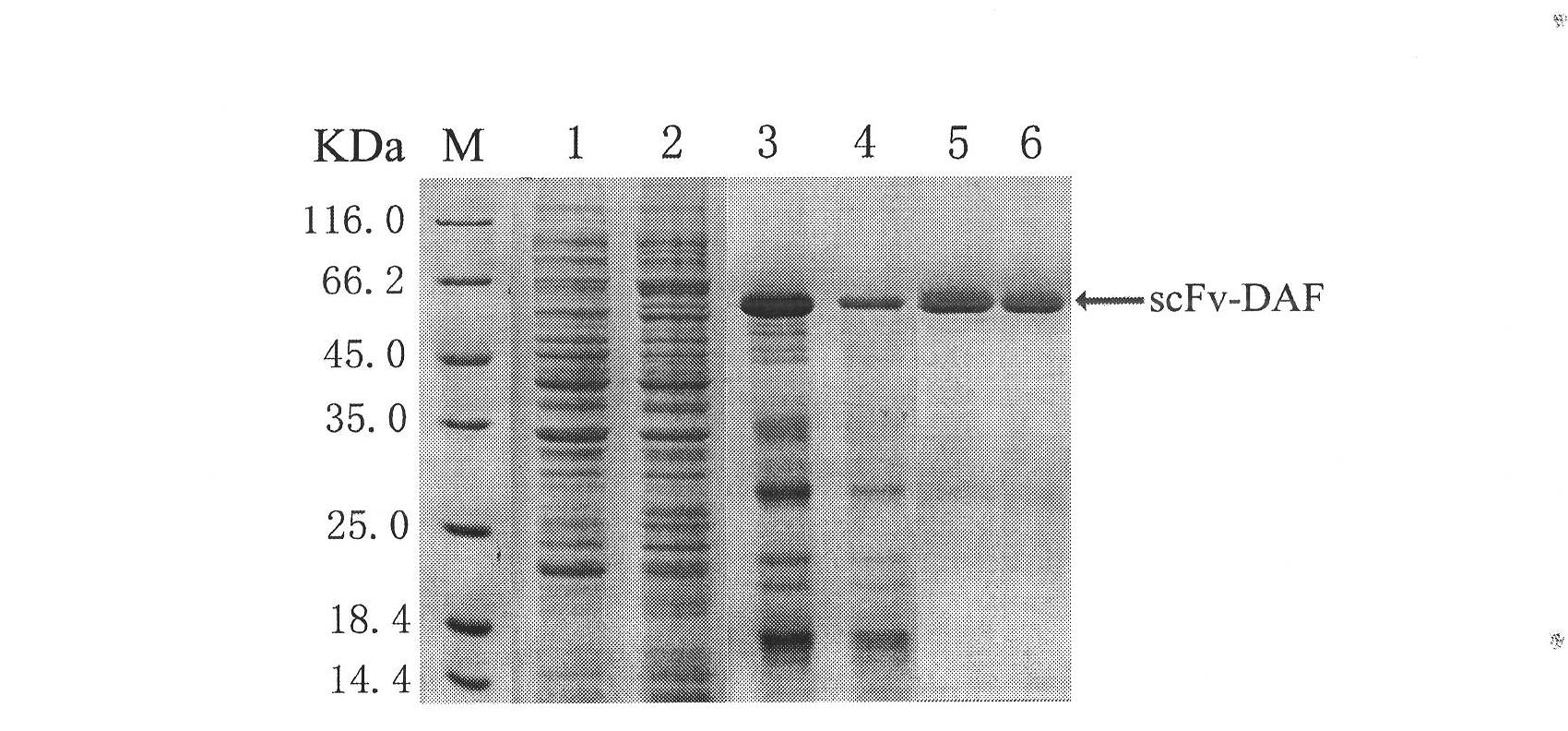
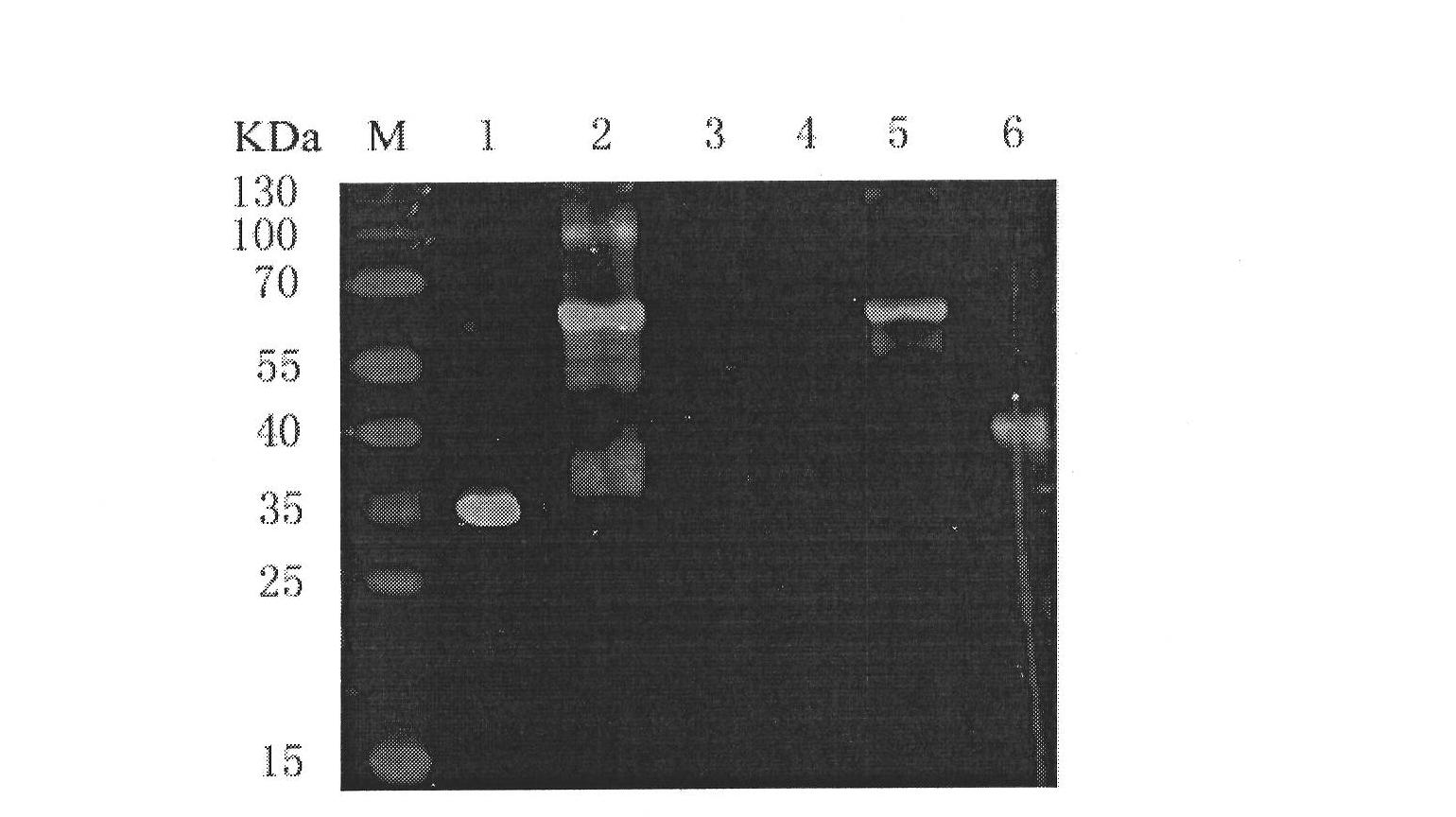
[0014] Specifically, the preparation of the fusion protein (scFv-DAF) of the above-mentioned single-chain antibody specifically binding to the acetylcholine receptor and the complement regulatory factor (DAF) can be carried out according to the following steps:
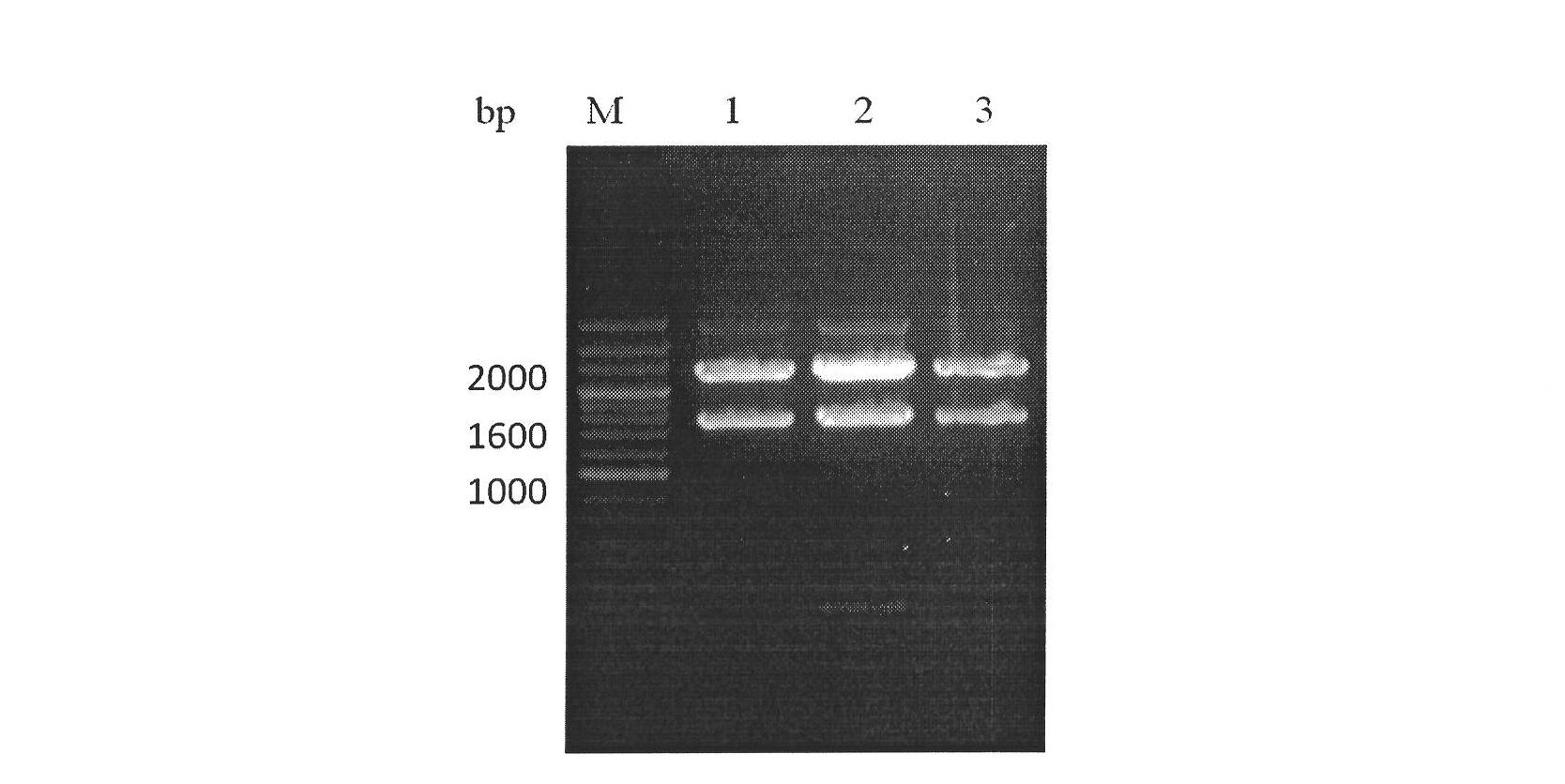
[0015] 1. Cloning of scFv-DAF gene
[0016] Using the plasmid pHEN1 containing anti-human AChR scFv1956# and the plasmid RD37 (NCBI AccessionNo.AB026902) containing DAF cDNA as templates, primers 1, 2 and primers 3 and 4 were used as upstream and downstream primers, respectively, using Primer STAR TM HS DNA polymerase amplifies the structural gene of scFv1956# and the SCR1-4 fragment of DAF. Primers 2 and 3 added a connecting sequence (G 4 S 1 ) 3 , Primers 1 and 4 respectively added two restriction sites NdeI and BamHI.
[0017] Primer 1 (scFv5' primer): The underlined part is the added NdeI restriction site.
[0018] 5' TTT CATATG CAGGTCCA ATTTGTAGAG3';
[0019] Primer 2 (connecting strand 3' primer): The s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com