Improved methods and compositions for F-18 labeling of proteins, peptides and other molecules
An F-18, protein technology, applied in the direction of peptide/protein isotope introduction, drug combination, preparations for in vivo tests, etc., can solve the problems of burden and long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
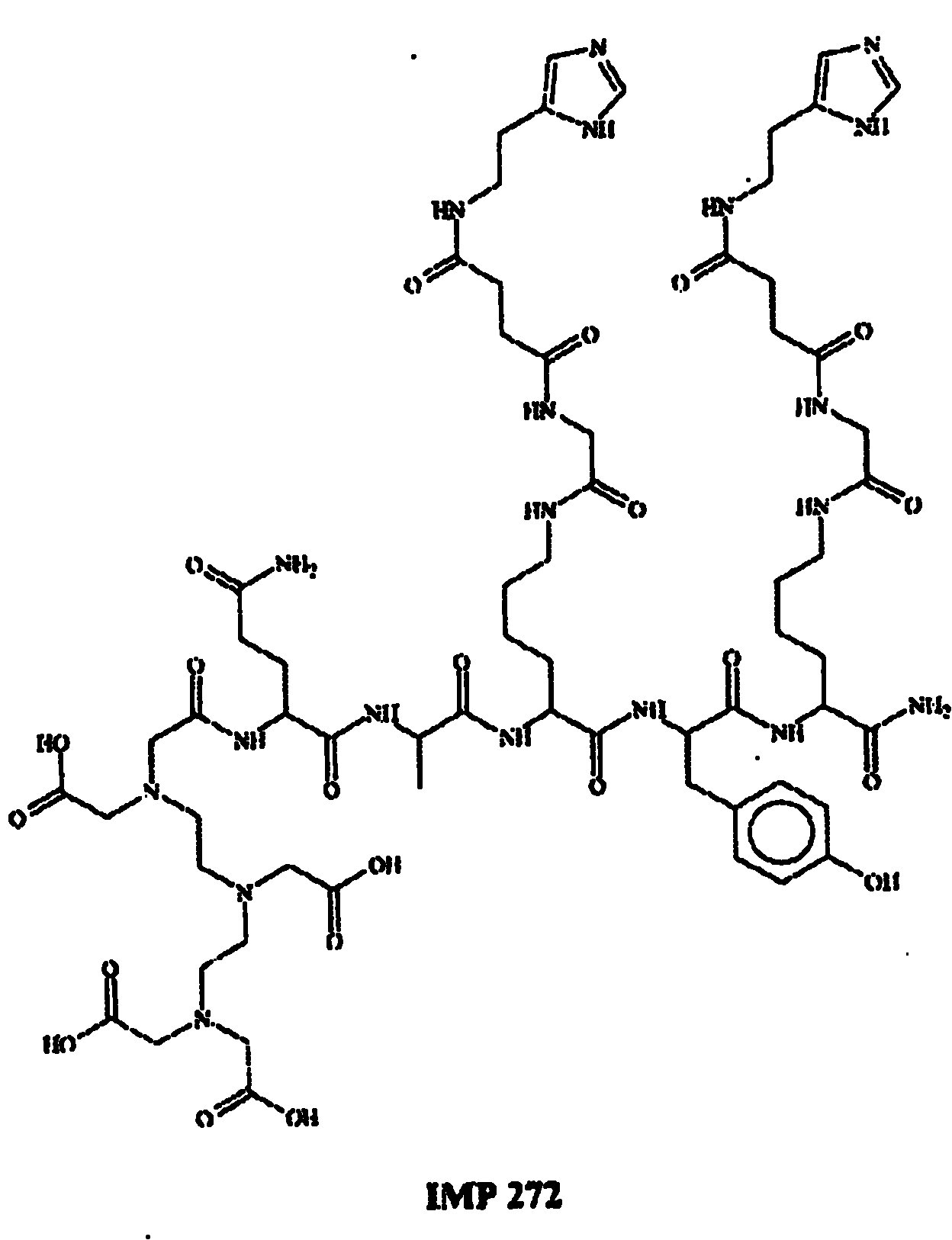
[0106] Example 1. F-18 Labeling of Peptide IMP272
[0107] The first peptide used was IMP272:
[0108] DTPA-Gln-Ala-Lys(HSG)-D-Tyr-Lys(HSG)-NH 2 M H + 1512
[0109] Acetate Buffer Solution - Acetic acid 1.509g was diluted in ~160ml water and the pH was adjusted by adding 1M NaOH then diluted to 250ml to obtain a 0.1M solution at pH 4.03.
[0110] Aluminum acetate buffer solution: the aluminum solution is passed through 0.1028g of AlCl 3 The hexahydrate was prepared by dissolving in 42.6 ml DI water. A 4ml aliquot of the aluminum solution was mixed with 16ml of a 0.1M NaOAc solution (pH 4) to provide a 2mM Al stock solution.
[0111] IMP 272 acetate buffer solution: peptide 0.0011g, 7.28×10 -7 molIMP 272 was dissolved in 364 μL of 0.1 M pH 4 acetate buffer solution to obtain a 2 mM stock solution of the peptide.
[0112] F-18 labeled IMP 272: 3 μL aliquots of aluminum stock solution in REACTI-VIAL TM And mixed with 50 μL of F-18 (original sample) and 3 μL of IMP 272 sol...
Embodiment 2
[0136] Example 2. Immunoreactivity of F-18IMP 272
[0137] Peptides (16 μL of 2 mM IMP 272, 48 μg) were labeled with F-18 and analyzed for antibody binding by size exclusion HPLC. Size exclusion HPLC showed that the peptide bound hMN-14x679 but not to the irrelevant bispecific antibody hMN-14x734 (not shown).
Embodiment 3
[0138] Example 3. IMP 272F-18 Labeled with Other Metals
[0139] ~3 μL aliquots of metal storage solution (6×10 -9 mol) was placed in a polypropylene conical tube and mixed with 75 μL F-18 (original sample), incubated at room temperature for ~2 minutes, and then mixed with 20 μL of 2 mM (4×10 -8 mol) IMP 272 solution mixed. The solution was heated in a heater at 100° C. for 15 minutes and analyzed by reverse phase HPLC. IMP272 was labeled (not shown) with indium (24%), gallium (36%), zirconium (15%), lutetium (37%) and yttrium (2%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com