Preparation of Everolimus
A technology of rapamycin and eluent, applied in the field of preparation of everolimus, can solve the problems of low yield, high cost, unsuitable for industrialized production and the like, and achieve the effect of reducing cost and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
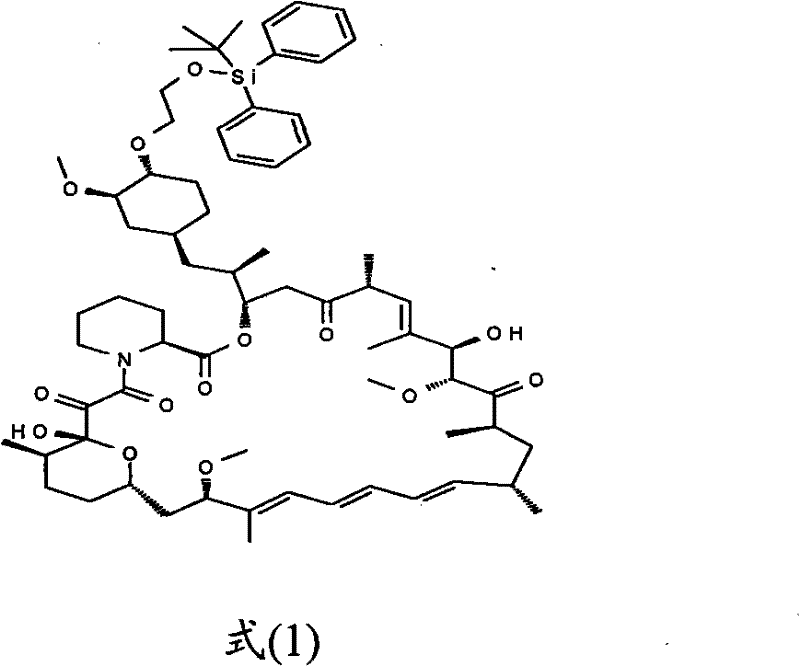
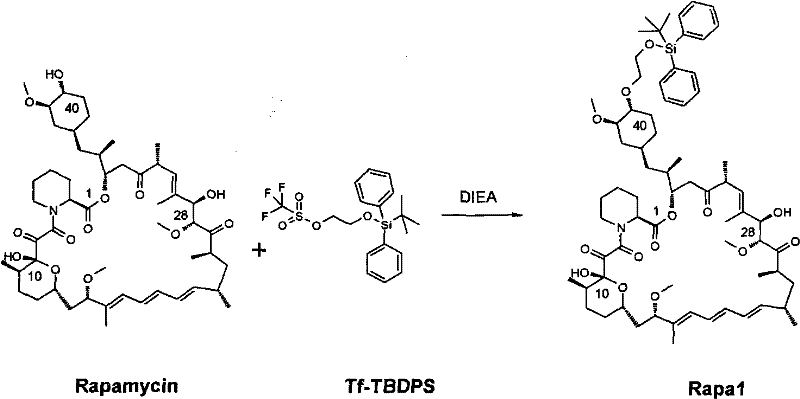
[0019] Embodiment 1. The preparation of intermediate
[0020] In a 100ml single-necked flask, add 4.61g of rapamycin and 8.64g of 2-(tert-butyldiphenylsilyl)oxyethyl trifluoromethanesulfonate respectively, then add 80ml of toluene solvent, room temperature Stirring, the solution is a milky white suspension. Then add 4.28g of diisopropylethylamine, heat up and stir, control the temperature between 50°C and 60°C, and react for 6 hours. After cooling to room temperature, the reaction solution was a light yellow clear solution. Add 100ml of ethyl acetate, wash the organic phase with 0.5M hydrochloric acid, saturated sodium bicarbonate solution and water respectively, and finally wash with saturated brine, dry with anhydrous magnesium sulfate for 6 hours, filter and concentrate under reduced pressure to obtain light yellow viscous liquid. Column chromatography, the eluent is petroleum ether and ethyl acetate (5:3), the intermediate components are separated and collected, and the...
Embodiment 2
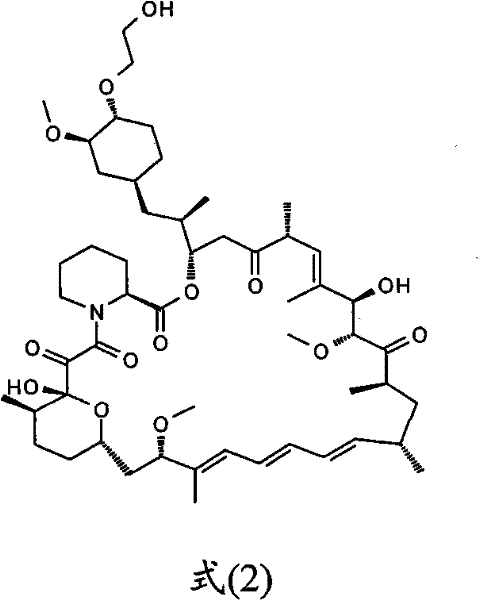
[0022] Example 2. Preparation of Everolimus
[0023] In a 100ml single-necked flask, 1.75g of the intermediate was dissolved in 60ml of tetrahydrofuran under cooling in an ice bath, and 6ml of pyridine hydrogen fluoride solution was added under stirring. The mixed solution was reacted at 0°C for half an hour, and then stirred overnight at room temperature. The reaction solution was poured into a mixed solution composed of 200ml ethyl acetate and 200ml saturated sodium bicarbonate solution, the organic phase was separated, and the aqueous phase was extracted 3 times with ethyl acetate (50ml / time). The organic phases were combined, washed with 1M hydrochloric acid, saturated sodium bicarbonate solution, water and saturated sodium chloride solution respectively, dried with anhydrous magnesium sulfate for 3 hours and filtered, and the filtrate was concentrated to obtain a yellow mucus. Column chromatography separation, ethyl acetate as eluent. The solvent was removed by evapor...
Embodiment 3
[0024] Embodiment 3. The recovery of rapamycin raw material and apply mechanically to prepare intermediate
[0025] In a 50ml single-necked flask, add the 1.00g rapamycin recovered from Example 1, then add 1.89g 2-(tert-butyldiphenylsilyl)oxyethyl trifluoromethanesulfonate, 15ml toluene And 0.94g diisopropylethylamine, repeat the method described in Example 1 to carry out reaction and aftertreatment. The intermediate components were concentrated under reduced pressure to obtain 0.35 g of foamy white solid (yield: 27.0%), and the raw material components were concentrated under reduced pressure to obtain 0.15 g of white solid rapamycin (recovery rate: 15%) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com