Method for manufacturing sustained release microsphere by solvent flow evaporation method
A technology of slow-release microspheres and water solvent, which is applied in the directions of pill delivery, endocrine system diseases, block delivery, etc. It can solve the problems of patients' fear and large size, and achieve the effect of inhibiting the initial excessive release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 6
[0042] Preparation method of emulsion containing ralin acetate
[0043] According to the content recorded in Table 1, 120 mg of goserelin acetate (manufacturer: Bachem, Switzerland) was added to 375 mg of water for injection, and dissolved by stirring to obtain a transparent aqueous phase.
[0044] Then, 1,250 mg of RG502H and 630 mg of RG503H (manufacturer: Boehringer Ingelheim) were used as a biodegradable polymer, 5.0 mg of Span 80 was used as a surfactant, and 5,000 mg of dichloromethane (manufacturer: Merck & Co. ) was stirred vigorously to dissolve, and after it became transparent, it was added into the aqueous phase dissolved with goserelin acetate and stirred vigorously to form an emulsion.
[0045] Table 1
[0046]
[0047] Prepare microspheres according to the type of co-solvent
[0048] According to the contents shown in Table 2, co-solvents were added to the above-prepared emulsions respectively and vigorously stirred. This solution was slowly poured into 0.5...
experiment example 1
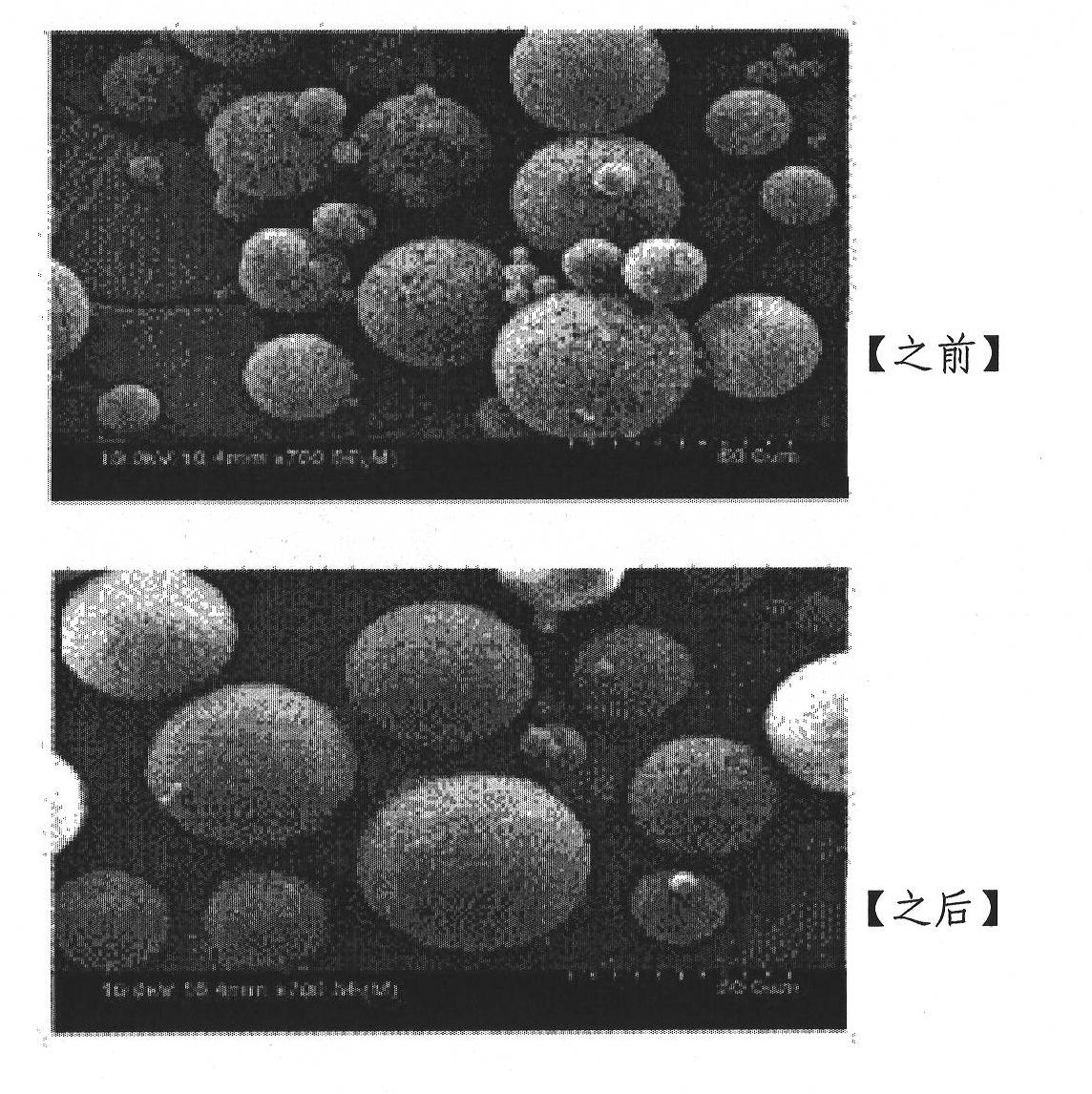
[0055] Experimental Example 1 Determination of the morphology of microspheres
[0056] In order to observe the surface of the microspheres, about 10 mg of microspheres were immobilized on an aluminum tube, and platinum coating was carried out for 3 minutes under a vacuum degree of 0.1 torr and a high voltage (10 kV), and loaded into a SEM (device name: Hitachi S-4800FE -SEM) and observe the particle surface by image analysis program.
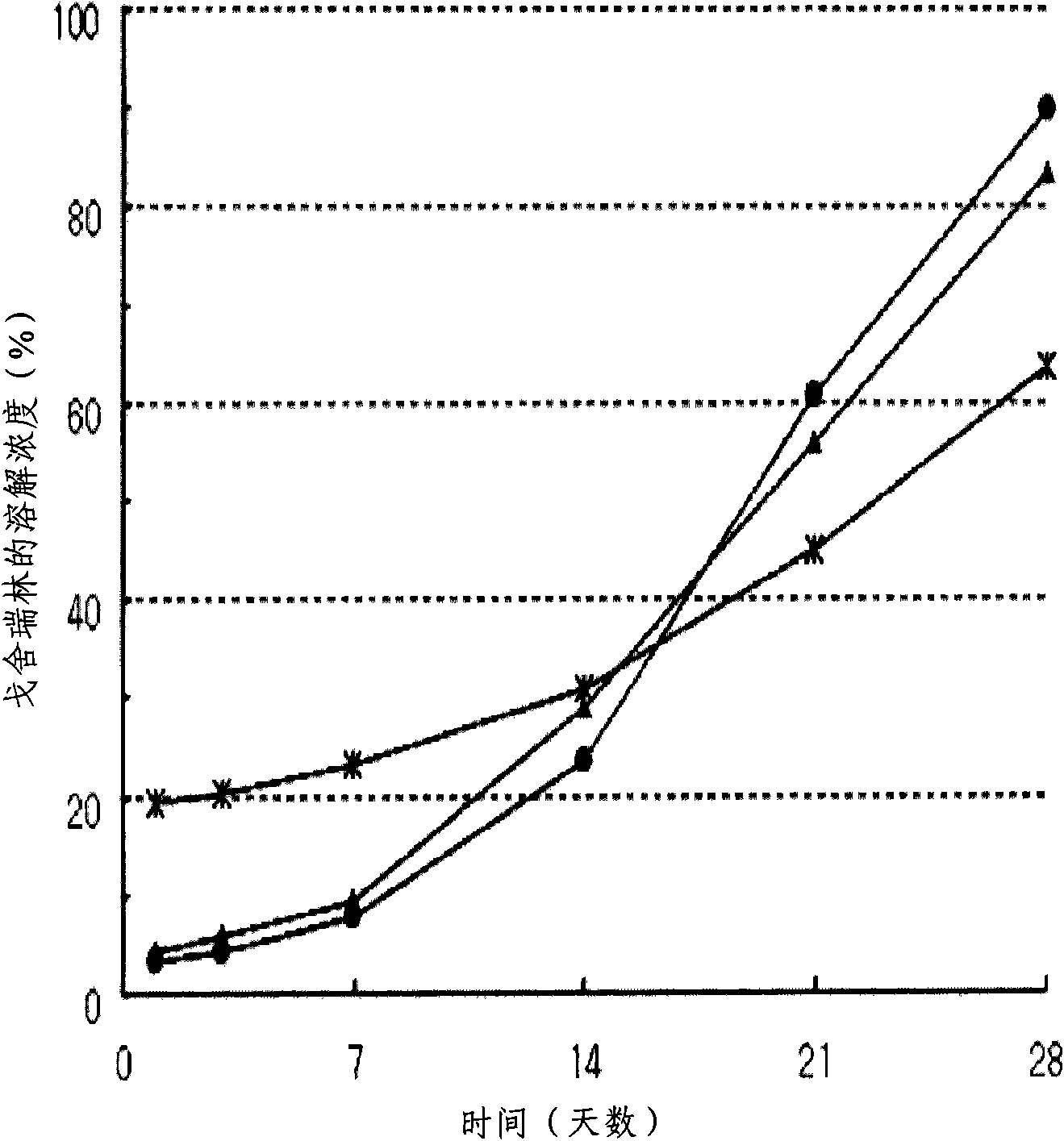
[0057] Measurement results such as figure 1 As shown, it was possible to confirm that the porosity of the surface was relatively reduced when the co-solvent was used.
experiment example 2
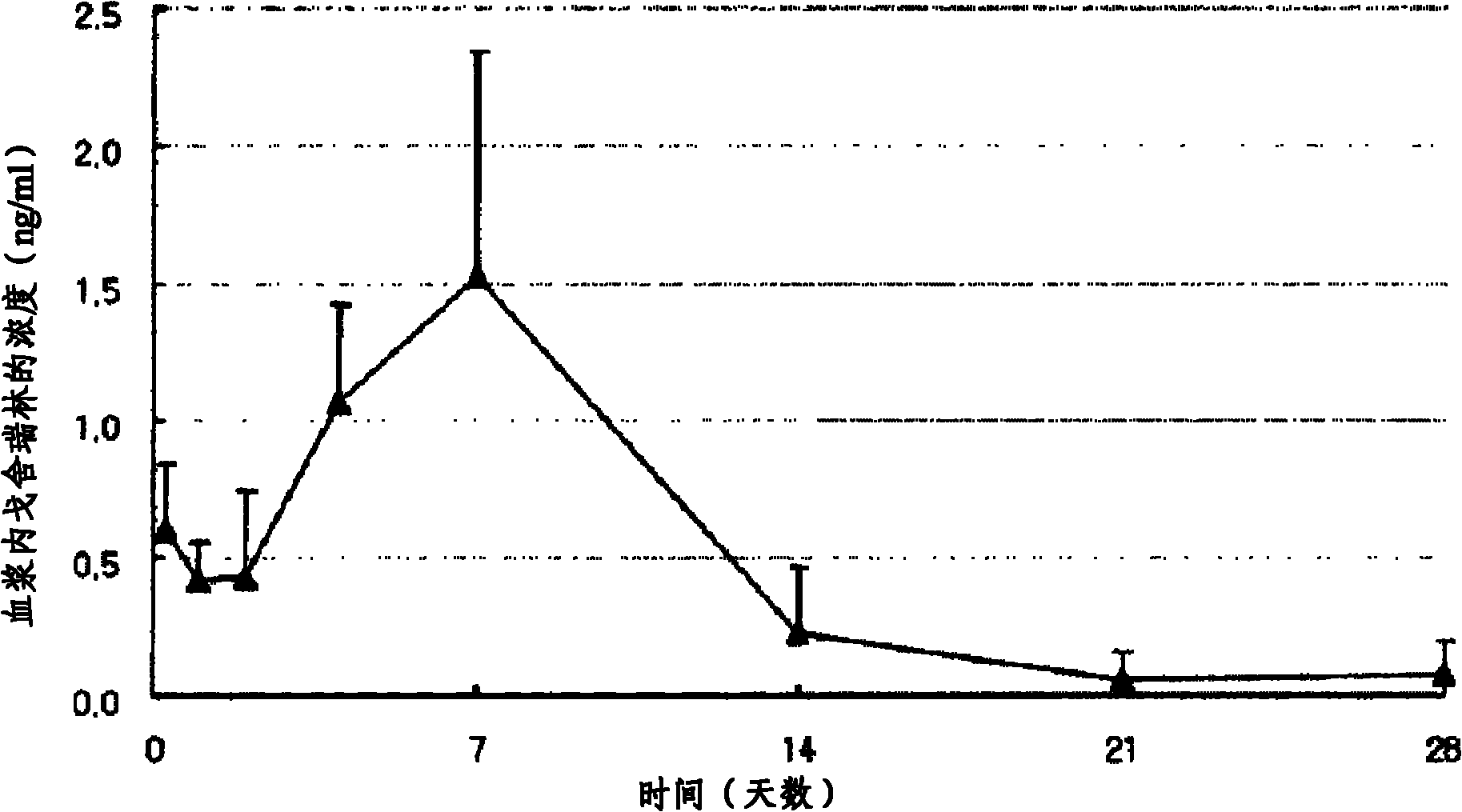
[0058] Experimental example 2 Determination of goserelin in microspheres
[0059] After adding 100 mg of microspheres to 25 ml of dimethylformamide (manufacturer: Merck) and completely dissolving them, filter them with a 0.45 μm syringe filter and use them as a reagent, and measure the concentration of goserelin encapsulated in the microspheres by HPLC. content. In the HPLC used this time, the chromatographic column is YMC C18ODS 5μm, 4.6x50mm, the sample volume is 10μl, and the detection wavelength is 280nm. The mobile phase was determined using phosphate buffer at pH 3.0.
[0060] As can be seen from the measurement results shown in Table 3, more than 90% of the added amount of goserelin was fully enclosed in the microspheres.
[0061] table 3
[0062]
[0063] Example 4
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com