Derivative of multi-target antitumor inhibitor 2-aminopyrrole-triazine and synthesis method thereof
An anti-tumor and inhibitor technology, applied in the field of cancer cells, can solve the problems of limited clinical application, toxic and side effects, etc., and achieve the effect of strong therapeutic effect, small side effects and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0051] 1. Synthesis method:
[0052] The raw materials used in the present invention all come from market sales.
[0053] Hereinafter "DMF" is dimethylformamide, "DMAP" is dimethylaminopyridine, "TBME" is methyl tert-butyl ether, "Me" is methyl, "TEA" is triethylamine, " "i-PrOH" is isopropanol, "DMSO" is dimethylsulfone, "DCM" is dichloromethane, "TLC" is thin-layer chromatography, and "MTBE" is methyl tert-butyl ether.
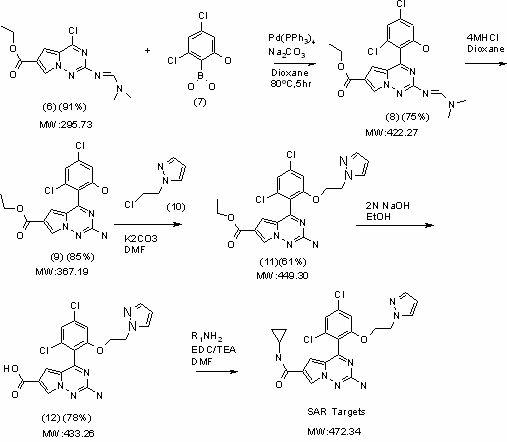
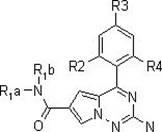
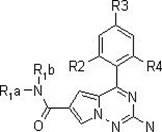
[0054] The following reaction steps illustrate a synthetic route of the present invention:
[0055]
[0056]
[0057] Specific instructions for each reaction step:
[0058] 1. Synthetic method of 1H-pyrrole-2,4 dicarboxylic acid (2) of compound diethyl ester:
[0059] First (4.56 g, 40 mmol) ethyl isocyanoacetate (1) and (9.2 g, 60 mmol) 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) were dissolved in 200 ml of tetrahydrofuran to form a solution, then add 80mmol of p-formaldehyde to the solution, and place the reaction mixture in an environment of 50±0.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com