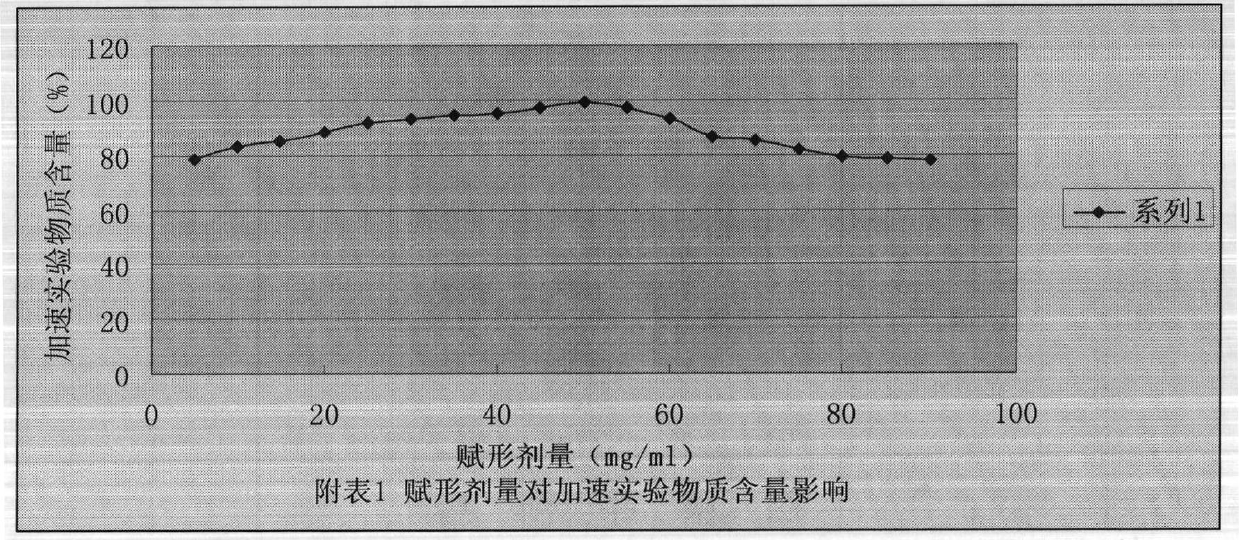
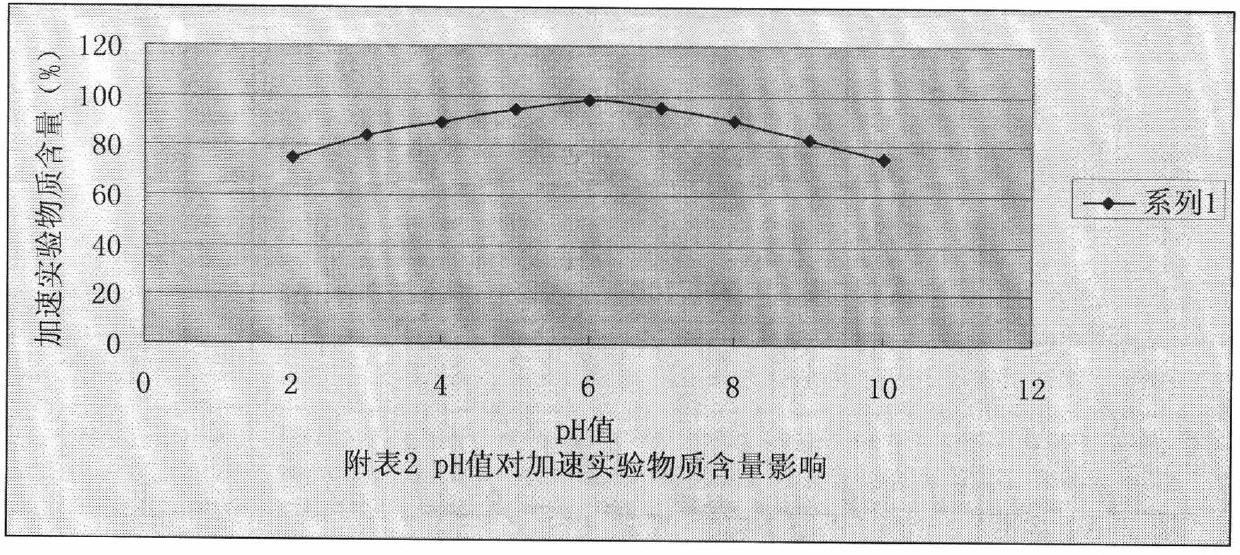
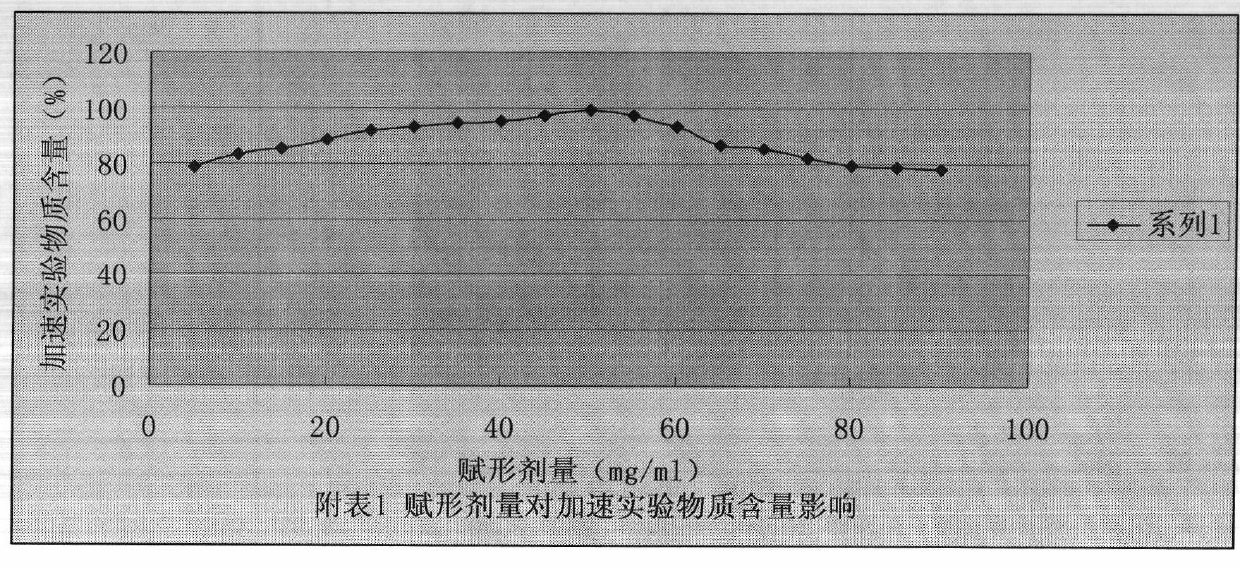
More stable nitrogen heterocyclic peptide preparation
A technology for injection preparations and excipients, which is applied in the field of azacyclohexyl peptide preparations, can solve problems such as harsh and unstable storage conditions, and hidden safety hazards for patients in drug circulation, and achieve the effect of increased stability, facilitating circulation and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Table 1-1 embodiment 1 composition formula
[0017] components
Dosage
42mg / ml
30mg / ml
20mg / ml
20mM
Appropriate amount, adjusted to pH 6.6
[0018] Its preparation process is as follows: in 2500ml beaker, add 75g sorbitol, 50g mannitol, 1750ml water, add caspofungin, be 42mg / ml to its final constant volume concentration, add sodium dihydrogen phosphate to its concentration and be 20mM, And the pH was adjusted to 6.6 with 1 N NaOH. Add water for injection to volume. Before filtering, add 12.5g of activated carbon to the injection, absorb pyrogen for 30 minutes under stirring, decarbonize and filter. The filtrate was filtered through a 0.22 μm titanium rod filter, and then sterilized and filtered through a 0.22 μm microporous membrane. Each bottle is filled into a 10ml glass vial with an amount of 1.25ml, freeze-dried, sto...
Embodiment 2- Embodiment 10
[0059] Embodiment 2-embodiment 10 formula is as follows
[0060]
[0061]
[0062]
[0063] The preparation process of embodiment 2-embodiment 10 is basically the same as that of embodiment 1, all are to add all excipients in 2500ml beaker earlier, 1750ml water, add caspofungin to its final constant volume concentration and be 42mg / ml , add buffer salt to its concentration, and adjust the pH to 6.6 with 1N NaOH, add water for injection to volume. Wherein in embodiment 10, benzyl alcohol and sodium thiosulfate are added after adding excipient. Before filtering, add 12.5g of activated carbon to the injection, absorb pyrogen for 30 minutes under stirring, decarbonize and filter. The filtrate was filtered through a 0.22 μm titanium rod filter, and then sterilized and filtered through a 0.22 μm microporous membrane. Each bottle is filled into a 10ml glass vial with an amount of 1.75ml, freeze-dried, stoppered, and capped to obtain caspofungin for injection. The lyophili...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com