Methods for preparing N-alkoxycarbonyl isothiocyanate and derivative thereof
A technology of alkoxycarbonyl isothiocyanate and its derivatives, which is applied in the field of preparation of isothiocyanate and its derivatives, can solve the problems of low reaction efficiency, difficulty in catalyst removal, high catalyst toxicity, etc., and achieve catalytic High efficiency, non-toxic catalyst, and less waste water generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1N-ethoxycarbonyl isothiocyanate
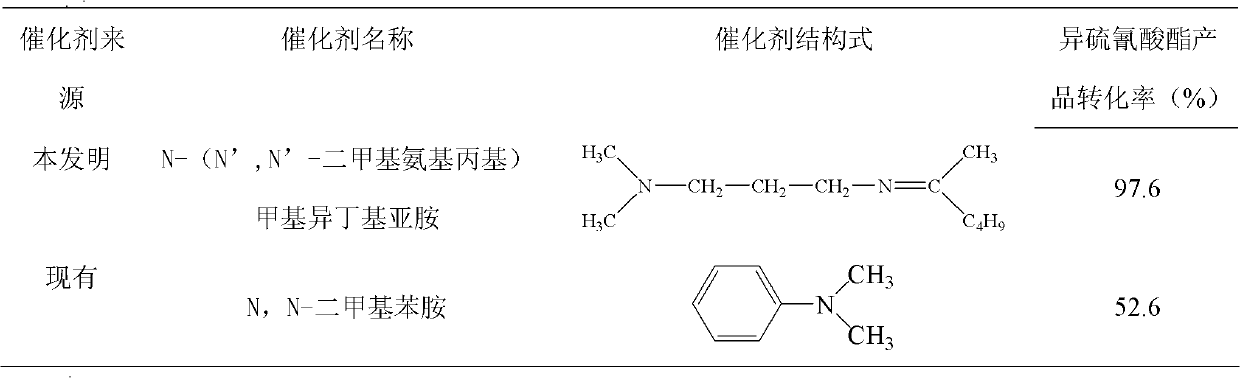
[0036]2.5 parts of N-(N', N'-dimethylaminopropyl) methylisobutylimine with a purity of 99% were dissolved in 200 parts of cyclohexane, and 45.3 parts of thiocyanic acid with a purity of 98.5% were added Sodium, the mixture was cooled to a temperature of 0°C to 5°C under stirring. 54.3 parts of ethyl chloroformate with a purity of 98.5% was added to the reaction mixture under stirring in this temperature range, and the reaction was stirred for 3 hours, and the reaction was completed. Add 100 parts of water to dissolve the precipitated sodium chloride generated by the reaction, and separate the liquid to remove the water phase. The oil phase was distilled off under reduced pressure to remove the solvent cyclohexane to obtain the desired N-ethoxycarbonyl isothiocyanate product. Analysis indicated a 98.4% product yield based on ethyl chloroformate.
Embodiment 2
[0037] The preparation of embodiment 2N-ethoxycarbonyl-O-ethylthiocarbonyl carbamate
[0038] The N-ethoxycarbonyl isothiocyanate product prepared according to Example 1 was added to 115 parts of ethanol while maintaining the temperature of the reaction mixture between about 25°C and about 45°C. The reaction mixture was then stirred at a temperature of about 40°C to 45°C for about 2 hours. The product obtained is an ethanol solution of N-ethoxycarbonyl-O-ethylthiocarbonylcarbamate. Analysis indicated a 95.6% product yield based on ethyl chloroformate.
Embodiment 3
[0039] The preparation of embodiment 3N-ethoxycarbonyl-O-isopropylthiocarbonyl carbamate (one pot method)
[0040] Add 28 parts of N-(N', N'-dimethylaminopropyl) cyclohexylimine with 98% purity to 453 parts of NaSCN with 98% purity in 1000 parts of acetone solution, then keep the temperature under stirring Between 10°C and about 25°C. 550 parts of 98.5% ethyl chloroformate were added to the reaction mixture at this temperature range with stirring. After the addition step, the reaction mixture was stirred at about 10°C to about 25°C for 4 hours to give N-ethoxycarbonyl isothiocyanate. Then maintain the reaction temperature between 10°C and 25°C, and add 491 parts of isopropanol under stirring. After the addition is complete, the reaction is continued with stirring for about 3 hours at a temperature of 45° C. to 55° C., and the reaction ends. The obtained mixture was washed with water, separated to remove the water phase, and the remaining acetone solvent was distilled off un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com