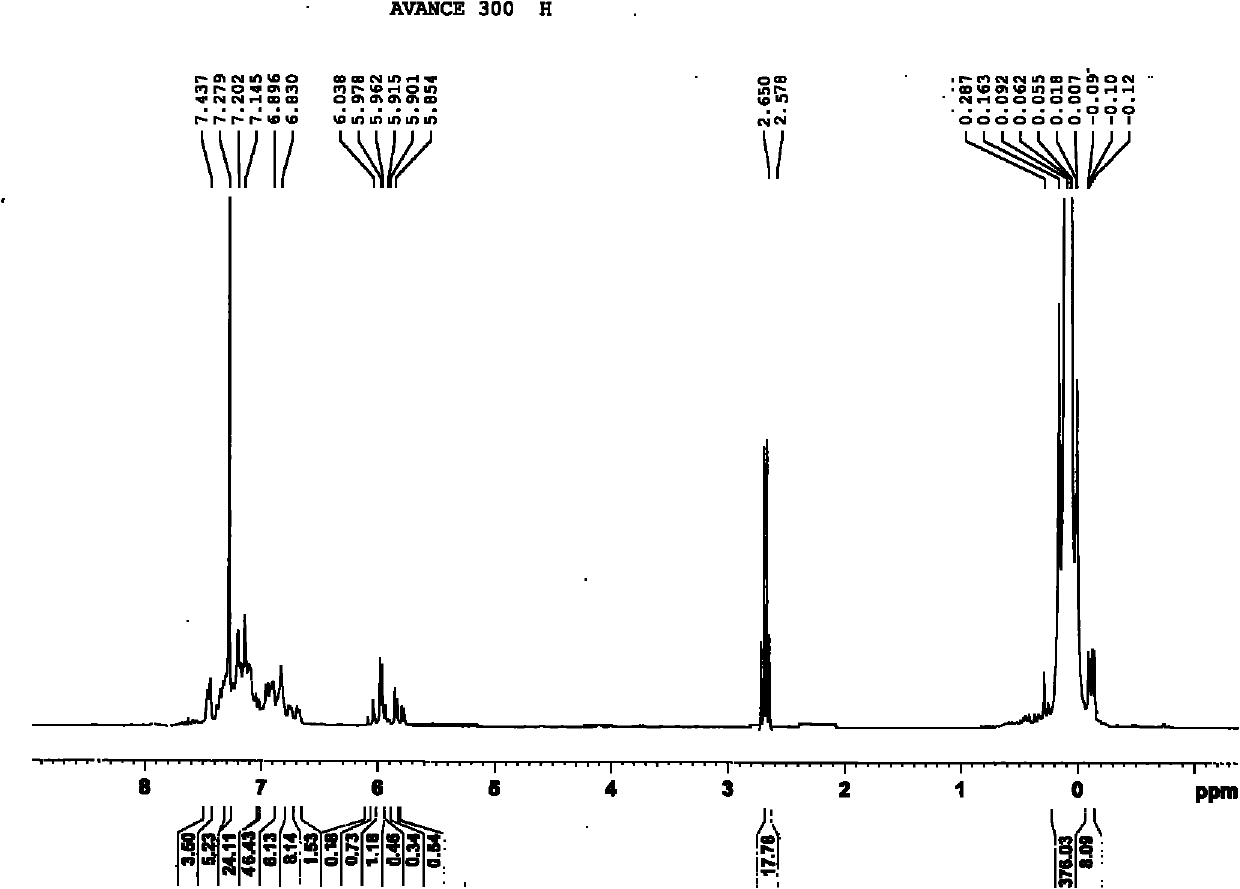
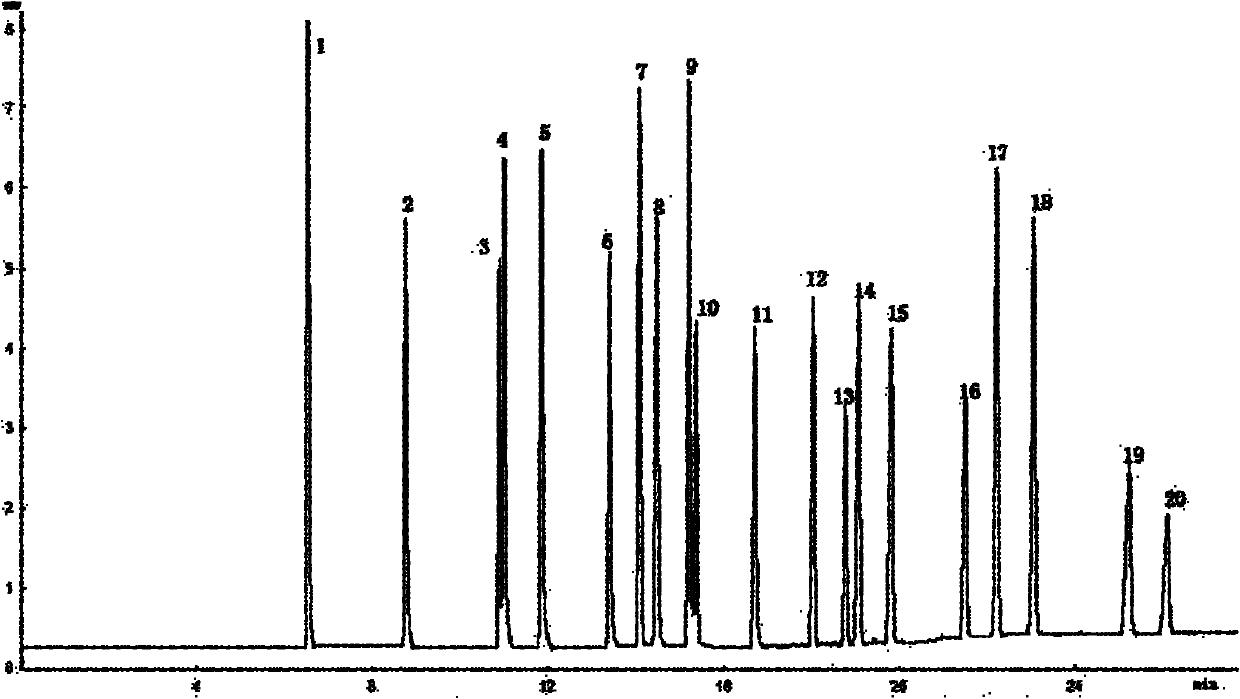
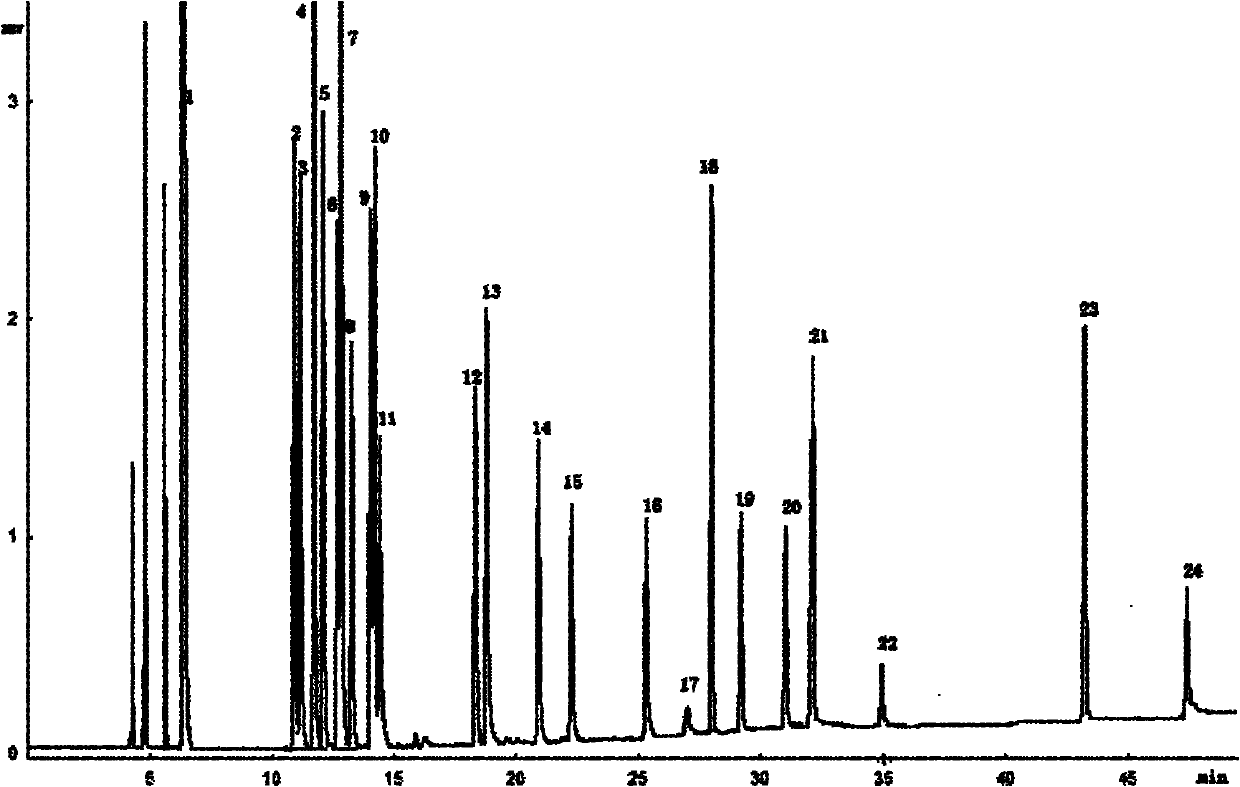
2-methyl-3,4-diphenyl phenyl-grafted polysiloxane and preparation method and application thereof
A technology of diphenylphenyl and polysiloxane, which is applied in the application field of gas chromatography stationary phase, can solve the problems of inability to meet the requirements of separation of complex mixture samples, and achieves excellent separation selectivity, excellent heat resistance and chromatographic properties. Separation of selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 2-Methyl-3, the preparation of 4-diphenylphenyl grafted polysiloxane, the specific preparation method is as follows:
[0036] (1) Synthesis of 2-methyl-3,4-diphenylcyclopentadienone:
[0037] 1. 4-hydroxyl-2 (5)-methyl-3, the synthesis of 4-diphenyl-cyclopent-2-enone: add 21.0g (0.1mol) benzil in 500ml round bottom flask, 18.0ml ( 0.2mol) butanone, 1.3g potassium hydroxide and 250.0ml absolute absolute ethanol, and electromagnetically stirred at room temperature for 24h. The reaction solution was poured into a beaker containing 1000ml of water, stirred to disperse the product, left to stand for 2 hours, and filtered with suction to obtain a white powder. It was recrystallized in a mixed solution of ethanol / water and fully dried to obtain 4-hydroxy-2(5)-methyl-3,4-diphenyl-cyclopent-2-enone as white crystals.
[0038] ② Synthesis of 2-methyl-3,4-diphenylcyclopentadienone: 25.0g (0.095mol) 4-hydroxyl-2(5)-methyl-3,4-diphenyl-cyclopentadienone Put 2-enone into a dry 100...
Embodiment 2
[0045] 2-Methyl-3, the preparation of 4-diphenylphenyl grafted polysiloxane, the specific preparation method is as follows:
[0046] (1) Synthesis of 2-methyl-3,4-diphenylcyclopentadienone:
[0047] 1. 4-hydroxyl-2 (5)-methyl-3, the synthesis of 4-diphenyl-cyclopent-2-enone: add 21.0g (0.1mol) benzil in 500ml round bottom flask, 18.0ml ( 0.2mol) butanone, 1.3g potassium hydroxide and 250.0ml absolute absolute ethanol, and electromagnetically stirred at room temperature for 24h. The reaction solution was poured into a beaker containing 1000ml of water, stirred to disperse the product, left to stand for 2 hours, and filtered with suction to obtain a white powder. It was recrystallized in a mixed solution of ethanol / water and fully dried to obtain 4-hydroxy-2(5)-methyl-3,4-diphenyl-cyclopent-2-enone as white crystals.
[0048] ② Synthesis of 2-methyl-3,4-diphenylcyclopentadienone: 25.0g (0.095mol) 4-hydroxyl-2(5)-methyl-3,4-diphenyl-cyclopentadienone Put 2-enone into a dry 100...
Embodiment 3
[0054] 2-Methyl-3, the preparation of 4-diphenylphenyl grafted polysiloxane, the specific preparation method is as follows:
[0055] (1) Synthesis of 2-methyl-3,4-diphenylcyclopentadienone: ① 4-hydroxyl-2(5)-methyl-3,4-diphenyl-cyclopentadienone Synthesis: 21.0g (0.1mol) of benzil, 18.0ml (0.2mol) of butanone, 1.3g of potassium hydroxide and 250.0ml of absolute ethanol were added to a 500ml round bottom flask, and electromagnetically stirred at room temperature for 24h. The reaction solution was poured into a beaker containing 1000ml of water, stirred to disperse the product, left to stand for 2 hours, and filtered with suction to obtain a white powder. It was recrystallized in a mixed solution of ethanol / water and fully dried to obtain 4-hydroxy-2(5)-methyl-3,4-diphenyl-cyclopent-2-enone as white crystals.
[0056] ② Synthesis of 2-methyl-3,4-diphenylcyclopentadienone: 25.0g (0.095mol) 4-hydroxyl-2(5)-methyl-3,4-diphenyl-cyclopentadienone Put 2-enone into a dry 100ml round-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com