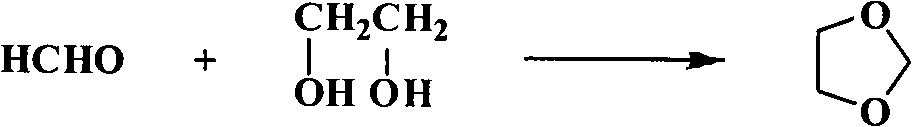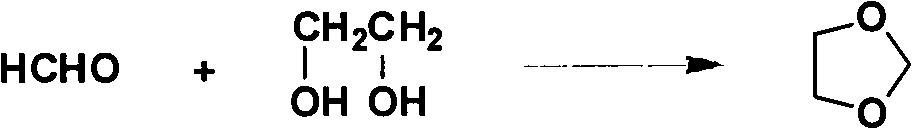New method for preparing 1,3-dioxolane by catalytic condensation
A technology of dioxolane and catalytic condensation, applied in the direction of organic chemistry, can solve the problems of easy pollution of the environment, strong corrosion, and use restrictions, and achieve the effects of high catalytic efficiency, low equipment requirements, and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: the preparation of 1,3-dioxolane
[0021] 4g SiO 2 (100 mesh) and 50 g of 2% phosphotungstic acid aqueous solution were mixed and stirred at room temperature for 10 h, dried at 100 ° C for 12 h after air drying, and then activated in a muffle furnace at 220 ° C for 2 h. That is, a phosphotungstic acid catalyst with a loading capacity of 20% is obtained.
[0022] Mix 62.07g (1.00mol) of ethylene glycol, 108.0g (1.20mol) of paraformaldehyde and 3g of 20% loaded phosphotungstic acid catalyst, heat to 100°C for 3 hours, filter to remove the catalyst and unreacted trimeric Formaldehyde, the resulting liquid is distilled at atmospheric pressure, and the fraction at 71-75°C is collected to obtain the crude product of dioxolane. Distillate, obtained refined dioxolane 63.5g, content 98.5%.
Embodiment 2
[0023] Embodiment 2: the preparation of 1,3-dioxolane
[0024] 7g SiO 2 (100 mesh) and 100 g of 3% phosphotungstic acid aqueous solution were mixed and stirred at room temperature for 12 hours, dried at 120°C for 12 hours after air drying, and then activated in a muffle furnace at 230°C for 3 hours. That is to obtain a phosphotungstic acid catalyst with a loading capacity of 30%,
[0025] Mix 62.07g (1.00mol) of ethylene glycol, 135.0g (1.50mol) of paraformaldehyde and 3g of phosphotungstic acid catalyst with a loading capacity of 30%, heat to 100°C for 3 hours, filter to remove the catalyst and unreacted trimeric Formaldehyde, the resulting liquid is distilled at atmospheric pressure, and the fraction at 71-75°C is collected to obtain the crude product of dioxolane. Distillate, obtained refined dioxolane 65.0g, content 98.5%.
Embodiment 3
[0026] Embodiment 3: the preparation of 1,3-dioxolane
[0027] 6g SiO 2 (100 mesh) and 100 g of 4% phosphotungstic acid aqueous solution were mixed and stirred at room temperature for 13 hours, dried at 130°C for 12 hours after air drying, and then activated in a muffle furnace at 250°C for 3 hours. That is to obtain a phosphotungstic acid catalyst with a loading capacity of 40%,
[0028] Mix 62.07g (1.00mol) of ethylene glycol, 180.0g (2.00mol) of paraformaldehyde with 3.0g of 40% phosphotungstic acid catalyst, heat to 100°C for 3 hours, filter to remove the catalyst and unreacted three Polyoxymethylene, the obtained liquid is distilled under normal pressure, and the fraction at 71-75°C is collected to obtain the crude product of dioxolane. °C fraction, 68.5 g of refined dioxolane was obtained, with a content of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com