Application of family 3 cellulose binding domain serving as affinity tag for expression and purification of recombinant protein in eukaryote
A protein expression, eukaryotic technology, applied in the field of protein purification, can solve problems such as the influence of CBM cellulose affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
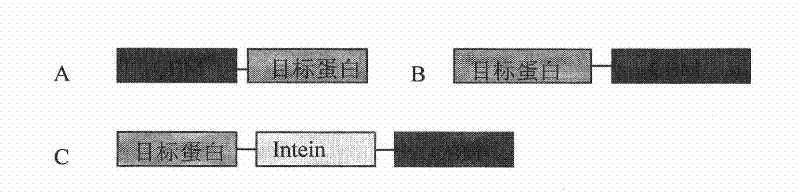
[0080] Fusion expression and purification of the cellulose-binding domain to EGFP.
[0081] EGFP is a green fluorescent protein, which is often used for molecular markers and protein localization. It is a common protein. In this example, this protein is used as an example to express in yeast by fusion with the cellulose-binding domain CBM3. Purification to demonstrate the ability and advantages of CBM3 expression and purification in affinity chromatography
[0082] Reagents and Strains:
[0083] All the reagents in the present invention are commercial reagent grade reagents. Microcrystalline cellulose Avicel PH105 (20 μm) was purchased from FMC Co (Philadelphia, PA). Escherichia coli Escherichia coli XL10 gold (purchased from Stratagene) was used as the host bacteria for DNA manipulation, and the Luria-Bertani (LB) medium containing 100 μg / mL ampicillin (purchased from Bio.Basic.Inu) was used to cultivate E.coli . Pichia pastoris (Pichia pastoris) KM71H (purchased from Inv...
Embodiment 2
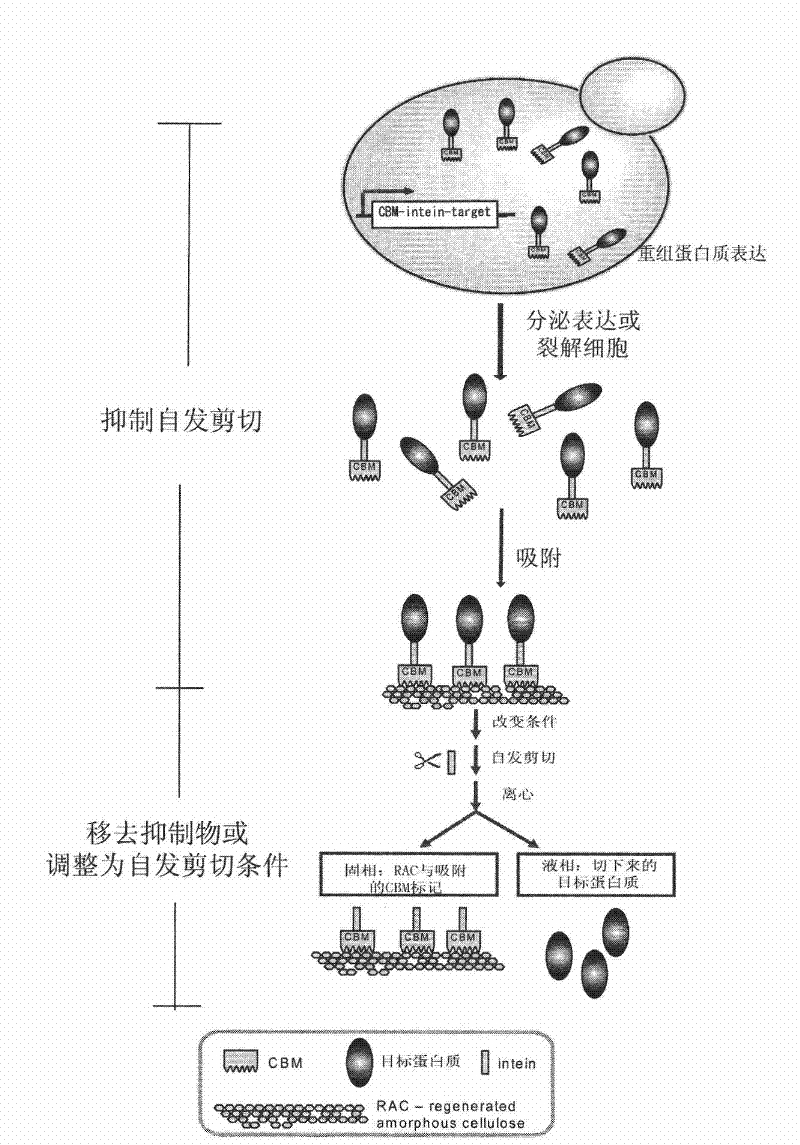
[0195] Fusion expression and purification of cellulose-binding domain with yeast intein VMA and EGFP, and pure EGFP protein without affinity tag was obtained by self-cleavage of intein.
[0196] In this example, EGFP is still taken as an example, and the cellulose-binding domain CBM3 and intein are fused and expressed in yeast, and purification is performed to demonstrate the advantages of intein in affinity chromatography for expressing target proteins fused with CBM3.
[0197] Reagents and Strains:
[0198] All reagents in this example are commercially available reagent grade reagents. Microcrystalline cellulose Avicel PH105 (20 μm) was purchased from FMC Co (Philadelphia, PA). Escherichia coli XL10 gold was used as the host bacteria for DNA manipulation, and Luria-Bertani (LB) medium containing 100 μg / mL ampicillin was used to cultivate E.coli. Pichia pastoris KM71H (purchased from Invitrogen) was used to express recombinant proteins. YPD (yeast extract, peptone, glucose...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com