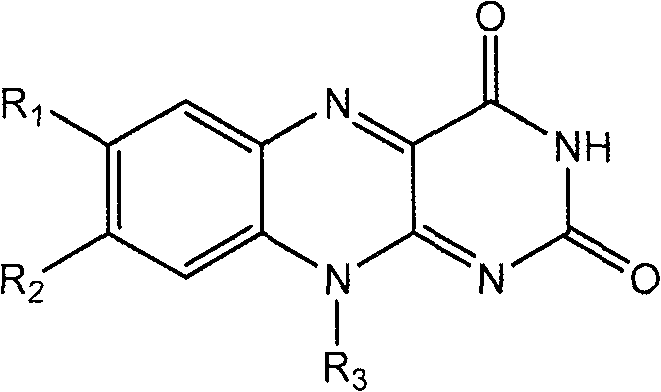
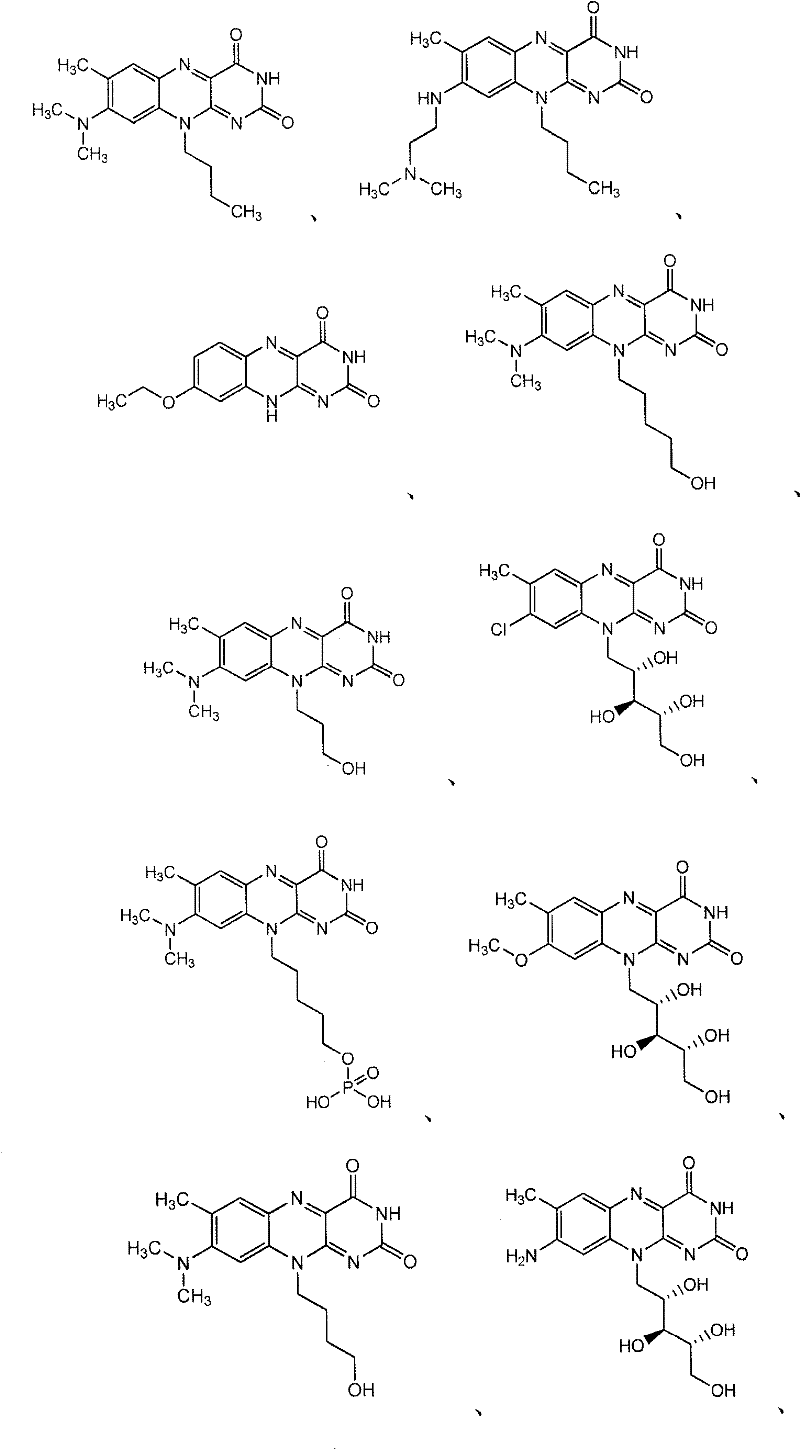
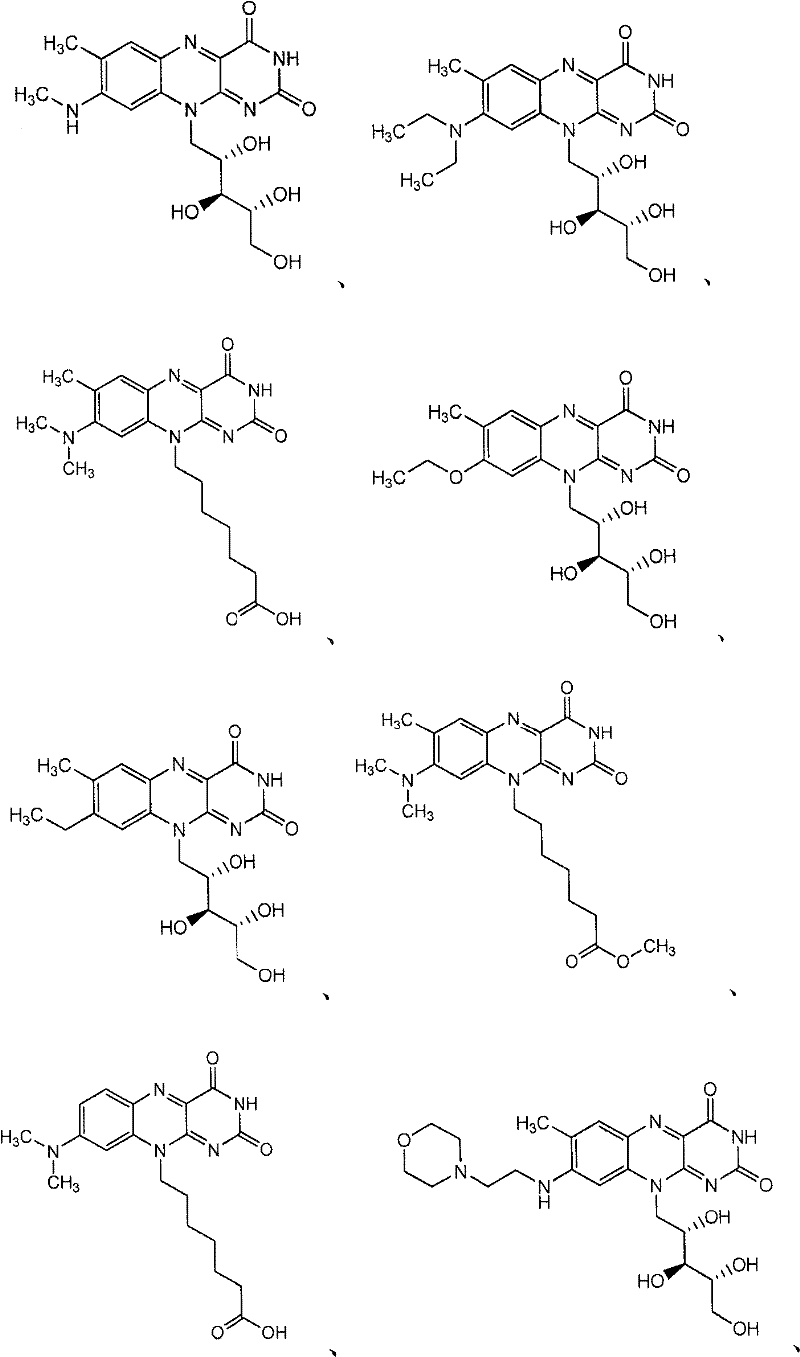
Flavin derivatives
A technology for compounds and prodrugs, applied in the field of flavin derivatives, can solve the problems of not identifying the type of nuclear switch, not identifying FMN, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0683] The preparation method of the compound of the present invention
[0684] Compounds of formula I, II, III, IV, V and VI and salts thereof can be prepared using methods described and practiced herein and methods analogous thereto and methods well known in the chemical arts. Such methods include, but are not limited to, those described below. In the descriptions of synthetic methods described herein, it is understood that all proposed reaction conditions, including choice of solvent, reaction atmosphere, reaction temperature, duration of experiments, and workup methods, are chosen to be standard for this reaction and should be readily understood by the present author. recognized by those skilled in the art. Thus, sometimes the reaction may need to be performed at elevated temperatures or for longer or shorter periods of time. Those skilled in the art of organic synthesis understand that the functional groups present on different parts of the molecule must be compatible w...
Embodiment 1
[0734] On-line probing assays, as described by Regulski and Breaker in "In-line probing analysis of riboswitches", (2008), Methods in Molecular Biology, Vol 419, pp 53-67, the contents of which are fully incorporated by reference, were performed with To estimate the dissociation binding constants for each of the ligands described herein interacting with FMN riboswitches amplified from the Bacillus subtilis genome. By in vitro transcription from PCR-generated templates and using the method described above [5'- 32 P]-tags were used to prepare pre-mRNA leaders (Regulski and Breaker, In-line probing analysis of riboswitches (2008), Methods in Molecular Biology Vol 419, pp 53-67). Dissolve approximately 5 nM labeled RNA precursor in 20 mM MgCl at 25 °C 2 , 50 mM Tris HCl (pH 8.3, at 25° C.) in the presence or absence of increasing concentrations of the respective ligands for 41 hours. The online cleavage products were separated by 10% polyacrylamide gel electrophoresis (PAGE), an...
Embodiment 1A
[0739] MIC determinations were performed in 96-well clear round bottom culture plates in a final volume of 100 μL according to the method established by the Clinical Laboratory Standards Institute (CLSI). Briefly, test compounds suspended in 100% DMSO (or another suitable solubilization buffer) are added to aliquots of media appropriate for the indicated pathogens to a total volume of 50 [mu]L. This solution was serially diluted 2-fold into successive tubes of the same medium to obtain the test compound concentration range suitable for the assay. To each dilution of test compound in medium was added 50 [mu]l of bacterial suspension from overnight culture growth in medium appropriate for the indicated pathogen. The final bacterial inoculum was approximately 10 5 -10 6 CFU / well. After 18-24 hours of growth at 37°C, the MIC was defined as the lowest concentration of an antimicrobial detectable with the naked eye that completely inhibited the growth of the microorganism relativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com