Application of ginkgo biflavonoids to preparation of medicine for preventing and treating asthma
A technology of ginkgo biflavanoids and compounds, which is applied in the field of medicine, can solve the problems of poor therapeutic effect and severe side effects of asthma drugs, and achieve safe and reliable long-term medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
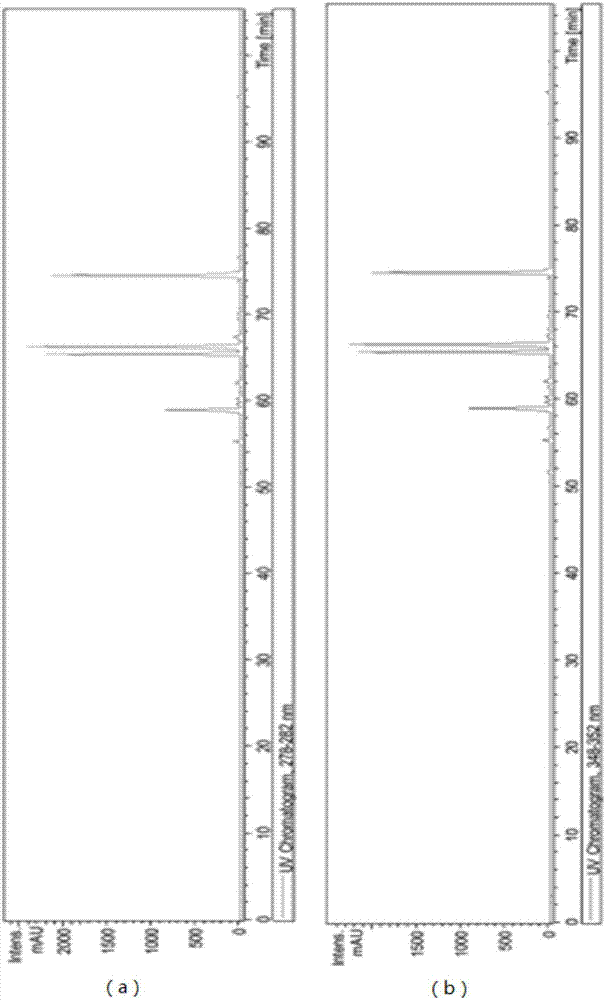
[0033] Embodiment 1: Extraction of Ginkgo biloba biflavonoids
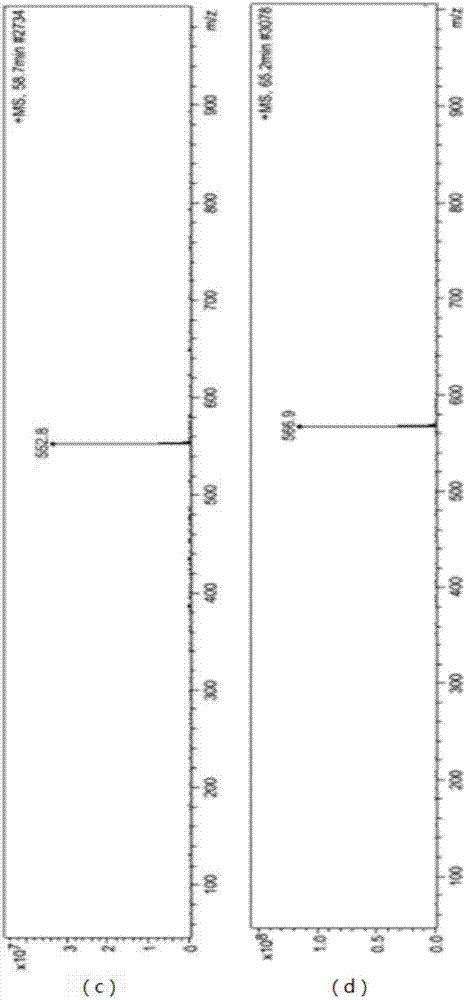
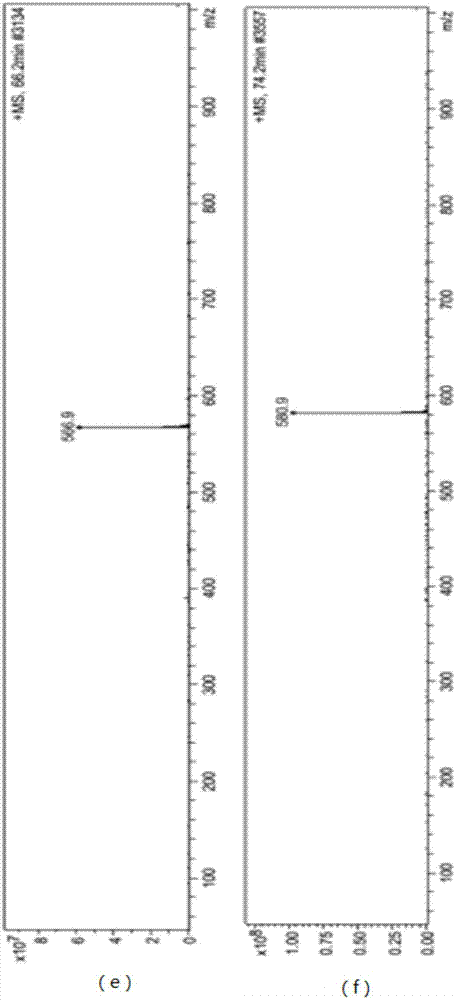
[0034] Take 500 g of dry ginkgo leaf powder, reflux extraction with 5 L of ether or ethyl acetate twice, recover the solvent, wash the initial extract with petroleum ether until it is colorless, put it on a silica gel column, elute with ethyl acetate, collect the eluent, and obtain Crude Ginkgo Biflavone. Use a certain proportion of pyridine and water to recrystallize the crude product, put it on a silica gel column, elute with a certain proportion of ethyl acetate and methanol, collect the four kinds of biflavones, combine them, and dry them to obtain the yellow dry Solid 7.5 g. The yellow dry solid containing four kinds of ginkgo biflavanoids was taken, dissolved in methanol, and the content detection and impurity analysis were carried out by LC-MS. The liquid phase conditions are as follows:
[0035]
[0036]
[0037] Liquid phase product model: 1100LC / MSD Trap, manufacturer: American Agilent Company, ...
Embodiment 2
[0041] Example 2: Effects of Ginkgo Biflavonoids on Pulmonary Inflammatory Cells in Asthmatic Mice
[0042] According to the asthma model experiment method in "Pharmacological Test Methods", an asthma mouse model was established. Animals: BALB / c mice, 6-8 weeks old, female, weighing 20-25 g. Ovalbumin sensitization, irritability, shortness of breath, abdominal muscle cramps, and fecal incontinence were positive reactions in mice.
[0043]The administration method is as follows: the doses of ginkgo biflavonoids (prepared according to Example 1, including four ginkgo biflavones, HPLC≥90%) are 10 mg / kg, 40 mg / kg, and 80 mg / kg, respectively. The prevention group was administered 2 hours before ultrasonic atomization; the treatment group was administered continuously for 6 days after 15-20 days of atomization.
[0044] Bronchoalveolar lavage fluid (BALF) total cell count and classification detection: After the experiment, the mice were sacrificed, the complete trachea and lungs w...
Embodiment 3
[0048] Example 3: Comparison of ginkgo diflavones and other active ingredients in ginkgo for the prevention and treatment of asthma
[0049] In this implementation, the asthma mouse model is the same as that in Example 2.
[0050] The administration grouping is: the positive control drug is dexamethasone (1mg / kg) (A, A'), and the drugs include: standard ginkgo extract (which contains ginkgo total flavonoid glycosides ≥ 24%, ginkgolide ≥ 6%) ( 10mg / kg (B, B'), 40mg / kg (C, C')) group; Ginkgo biloba (10mg / kg) group (D, D'); Ginkgolide (content ≥ 85%, 2.4mg / kg) group (E, E'); Ginkgo total flavonoid glycosides (content ≥ 85%, 9.6mg / kg) group (F, F'). Each drug is divided into two modes of administration, prevention and treatment. After centrifuging BLFA, take the supernatant, and use the ELASA kit to detect the inflammatory factors IL-4, IL-5, and IL-13 related to asthma.
[0051] For comparison results, see the attached Figure 4 , where the ordinate is the concentration of v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com