Expression and application of human papilloma viruses type 16 and 18 L1 proteins in inset cells
A technology of L1 protein and insect cells, applied in the fields of application, antiviral agents, viral antigen components, etc., can solve the problems of high cost and difficult to clearly prove the protective effect, and achieve the effect of increasing the expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The optimization of the gene sequence of the L1 protein of embodiment 1.HPV16 / 18
[0041] The amino acid sequences of the L1 proteins of the highly pathogenic HPV16 and HPV18 strains prevalent in my country are shown in SEQ ID NO.7 and 8, respectively.
[0042] Using the commercially available software OptimumGene TM The L1 gene sequence of HPV16 and HPV18 was optimized to increase the expression level of L1 gene in insect cells. Wherein, the optimized HPV16 L1 gene sequence is shown in SEQ ID NO.1; the optimized HPV18 L1 gene sequence is shown in SEQ ID NO.2.
Embodiment 2
[0043] Example 2. Construction of pVL16-L1 and pVL18-L1 transfection plasmids
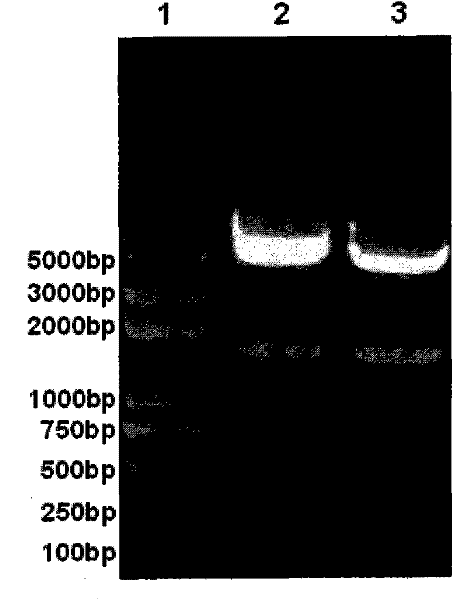
[0044] Using the primer pair 5'-CTC GAG GAA TTC AGG ATG CAA GTG ACG TTTATT-3' (SEQ ID NO.3) and 5'-A GAG CTC CCA TGG AGG TTA CAA CTTGCG TTT CTT-3' (SEQ ID NO.4 ), and the primer pair 5′-CTC GAG GAA TTCAGG ATG TGC TTG TAC ACG CGC-3′ (SEQ ID NO.5) and 5′-A GAG CTCCCA TGG AGG TTA TTT GCG AGC TCT CAC G-3′ (SEQ ID NO.5) NO.6), respectively amplifying artificially synthesized optimized L1 genes of HPV16 and HPV18 by PCR. The PCR reaction conditions were pre-denaturation at 95°C for 5 minutes; 30 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C for 45 seconds, and extension at 72°C for 2 minutes; finally, extension at 72°C for 10 minutes. After PCR amplification, the L1 gene fragments with Xho I and Nco I restriction endonuclease sites at both ends were obtained.
[0045] Use commercially available Xho I and Nco I (for example, purchased from Bao Biological Engineering (Dalian) Co., Lt...
Embodiment 3
[0047] Example 3. Preparation of recombinant baculovirus
[0048] The pVL16-L1 and pVL18-L1 recombinant plasmids were mixed with AcNPV genomic DNA (from the kit of BD Company of the United States, BD BaculoGold TM , 560129) together to co-transfect Sf-9 cells (purchased from Invitrogen, USA). After cultured at 27°C for 4 days, the volume of transfected cells was observed to increase under the microscope. After culturing for 5 days, the cell culture supernatants (containing recombinant viruses rBV16 and rBV18, respectively) were collected. Cell supernatants were inoculated into freshly cultured Sf-9 cells, and cultured at 27°C for 3 days. Take the culture supernatant and subculture again. Afterwards, take the culture supernatant, add an equal volume of 2×precipitation solution (containing 20% PEG6000, 1M NaCl), and mix overnight at 4°C. The mixture was then centrifuged at 13000 rpm for 30 minutes at 4°C, and the supernatant was discarded. The resulting pellet was resus...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com