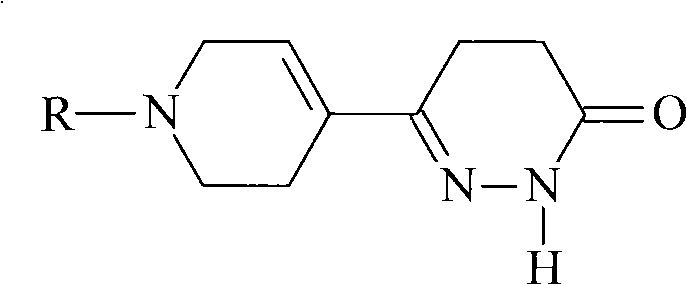
Pyridazinone derivative and synthetic method thereof
A technology of pyridazinone and its derivatives, applied in the field of compound and its synthesis, can solve the problem that pyridazinone compounds cannot meet the needs of clinical new drug development and selection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 synthetic compound I
[0061] Reagents: All reagents used are analytically pure;
[0062] Instruments: GF254 thin-layer chromatography plate, separating funnel, vacuum filter device, JA1203N thousandth balance (Shanghai Precision Scientific Instrument Co., Ltd.), 78-1 magnetic heating stirrer (Shanghai Nanhui Telecommunication Equipment Factory), EYELA Type rotary evaporator (Shanghai Ailang Instrument Co., Ltd.), SHB-IIIA circulating multi-purpose vacuum pump (Zhengzhou Great Wall Technology Industry and Trade Co., Ltd.), constant temperature water bath (Yuyao Dongfang Electrician Instrument Factory), ZD-2022 multi-function stirring Instrument (Tianjin Lihua Instrument Factory)
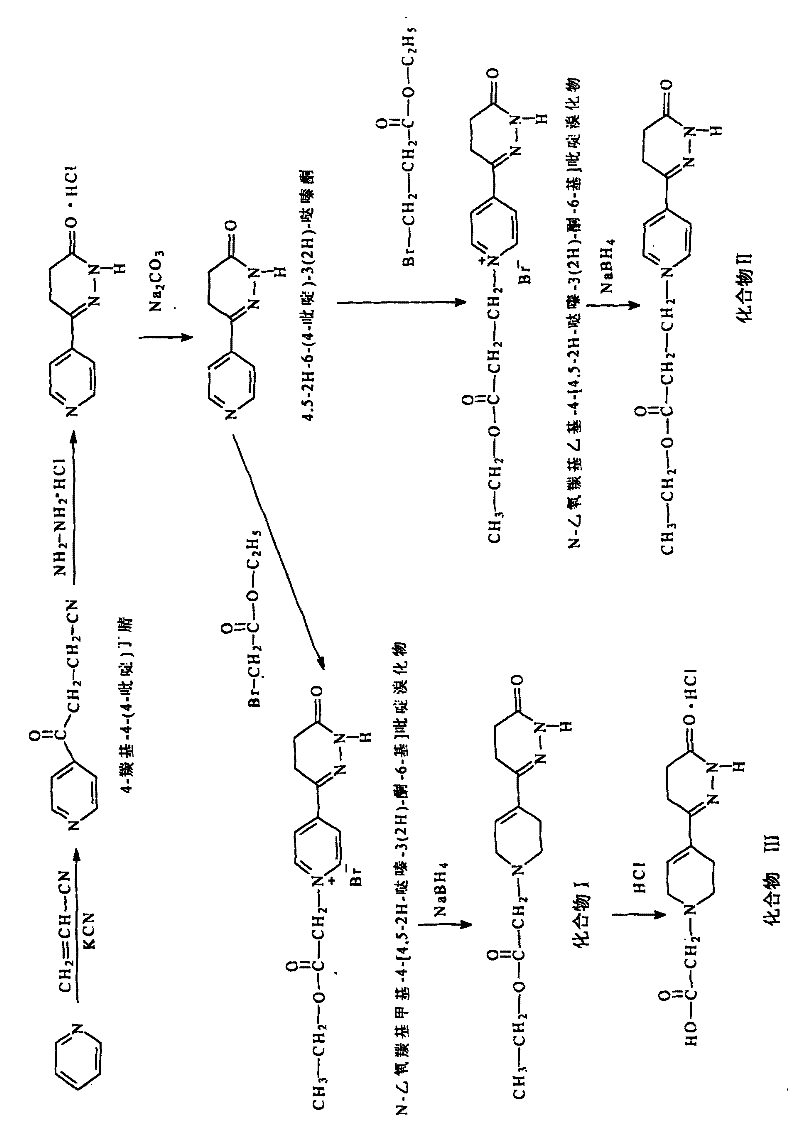
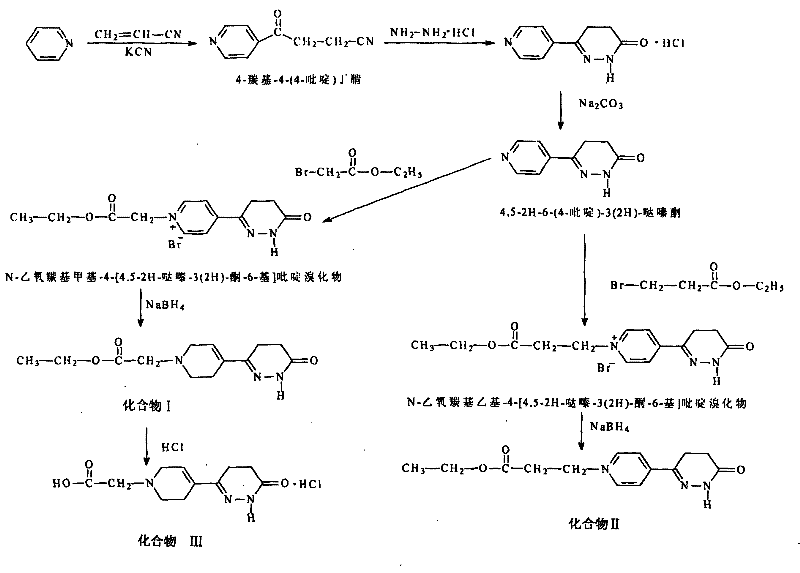
[0063] Synthetic route such as figure 1 Shown:
[0064] (1) Synthesis of 4-carbonyl-4(4-pyridine)butyronitrile
[0065] Get potassium cyanide 32.5g (0.5mol) and place in the there-necked flask, add acetonitrile 420mL and make it dissolve, 420mL contains the acetonitrile soluti...
Embodiment 2
[0077] Example 2 Preparation of compound II
[0078] Take the product N-ethoxycarbonylethyl-4-[4,5-pyridazin-3(2H)-one-6-yl]pyridine bromide 0.85g (2.5mmol), dissolve it in 15ml of methanol, and stir in an ice bath At the same time, 0.9 g of sodium borohydride was slowly added, and after the reaction was stable, it was stirred overnight at room temperature. The next day, the reaction solvent methanol was distilled off to obtain a white solid residue. Dissolve the residue with 20ml of dichloromethane and transfer it to a separatory funnel, wash twice with 10ml of distilled water, discard the water phase, add an appropriate amount of anhydrous magnesium sulfate to the organic phase and dry it, then evaporate to dryness under reduced pressure to obtain a light yellow substance, add 40ml of absolute ethanol was used for recrystallization; white crystals (compound II) were obtained by filtration with a yield of 30%.
[0079] The mass spectral data measured by the prepared compoun...
Embodiment 3
[0082] Example 3 Preparation of Compound III
[0083] Get 330mg (1.25mmol) of compound I prepared in Example 1 and add 20ml of 1mol / L hydrochloric acid to make it dissolve, heat to make it react, detect the reaction status with a TLC plate, when the reaction is nearly complete after 5.5h (there is no compound No. spot), stop the reaction; after filtration, the filtrate is evaporated to dryness under reduced pressure, the residue is recrystallized by adding 50ml of absolute ethanol, after freezing overnight, suction filtration obtains white crystal 4,5-dihydro-6-[(1-carboxymethyl) -1,2,5,6-tetrahydropyridin-4-yl]-3(2H)-pyridazinone hydrochloride (compound III) 85 mg, yield 25%.
[0084] The measured mass spectrum data of prepared compound III are:
[0085]
[0086]1 H-NMR (D 2 O): 4.15(s, 2H, 1-H); 4.13(d, 1H, 2-H); 3.69(t, 2H, 3-H); 2.59(t, 2H, 4-H); 6.94(s , 1H, 5-H); 2.61(t, 2H, 6-H); 3.07(t, 2H, 7-H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com