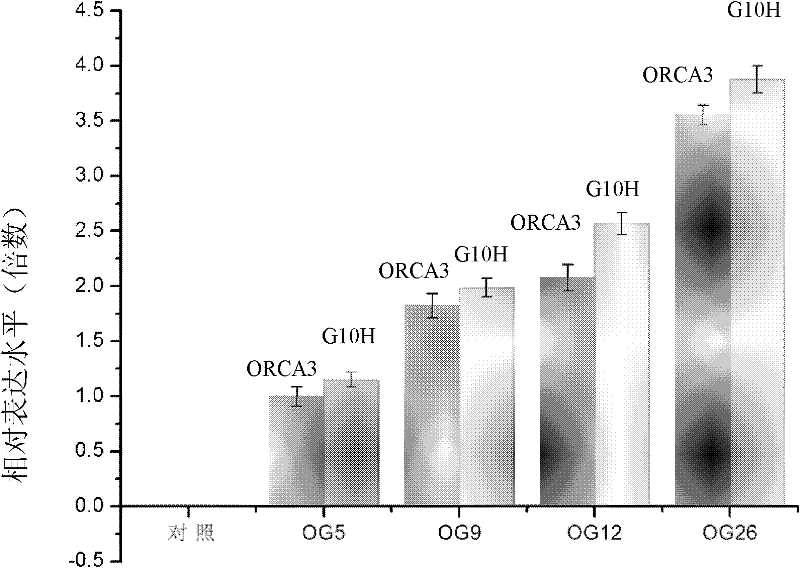
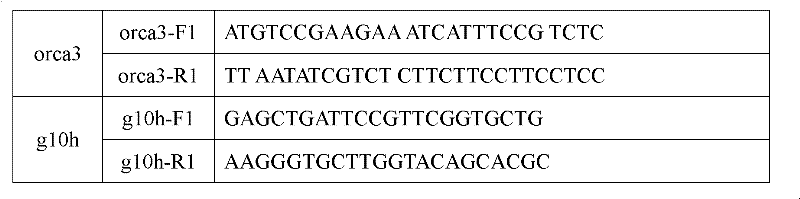
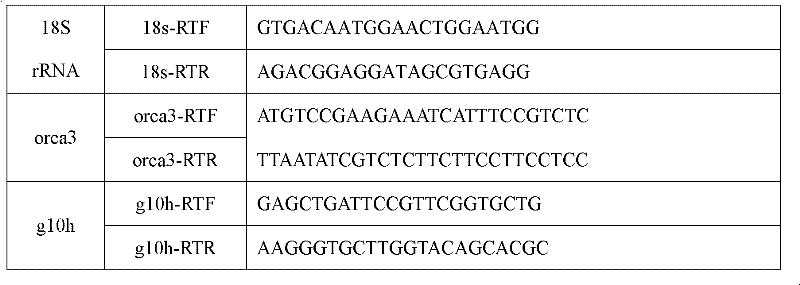Method for increasing content of camptothecin through co-transformation of double genes of transcription factor ORCA3 (Octadecanoie-responsive Cantharanthus AP2-doman protein 3) and key enzyme G10H (Geraniol 10-hydroxylase)
A transcription factor and key enzyme technology, applied in the biological field, can solve the problems of not significantly improving the yield of TIA metabolic end products, insufficient expression, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Obtaining of periwinkle CrORCA3 and CrG10H gene coding sequence
[0028] 1.1. Extraction of periwinkle total RNA and synthesis of cDNA first strand
[0029]Total RNA was extracted from periwinkle seedlings using the RNA prep pure plant kit provided by TIANGEN (see the instructions in the kit for the extraction steps). The fresh weight of periwinkle seedlings used to extract total RNA is about 0.1 g, and the DNA in the sample has been removed with DNase working solution during the extraction process. Measure the relative absorbance value of the extracted RNA on a spectrophotometer, and calculate the purity and concentration of the extracted RNA. After calculation based on the concentration of different RNA samples, the first-strand cDNA was synthesized with reverse transcriptase XL (AMV) using 0.5 μg RNA as the initial amount (for the operation steps, refer to the relevant instructions provided by Promega).
[0030] 1.2. Design of specific primers for CrOR...
Embodiment 2
[0032] Embodiment 2 Containing the construction of the plant expression vector of periwinkle CrORCA3 and CrG10H gene
[0033] 2.1. Intermediate vector pCAMBIA1304 + build
[0034] Using pBI121 and pCAMBIA1304 as materials, construct the plant expression vector pCAMBIA1304 + . Specifically, pBI121 and pCAMBIA1304 were digested with HindIII / EcoRI; the pBI121-GUS expression cassette and the large fragment of pCAMBIA1304 were recovered; ligation transformation was carried out, and single clone colonies were picked to extract plasmids for digestion verification. The results showed that the plant expression vector pCAMBIA1304 + The build was successful.
[0035] 2.2. Plant expression vector pCAMBIA1304 + - Construction of CrG10H
[0036] The successful pCAMBIA1304 constructed above + Basically, replace the GUS gene on it with the CrG10H gene cloned from periwinkle. Specifically, BamHI / SacI double enzyme cut pMD18T-CrG10H and pCAMBIA1304 + ; Recover CrG10H gene and pCAMBIA13...
Embodiment 3
[0040] Example 3 Agrobacterium rhizogenes mediated genetic transformation of CrORCA3 and CrG10H genes to obtain transgenic hairy roots
[0041] 3.1. Containing plant expression vector pCAMBIA1304 + -Acquisition of CrORCA3-CrG10H Agrobacterium rhizogenes Engineering Bacteria
[0042] The plant bivalent expression vector pCAMBIA1304 containing CrORCA3 and CrG10H gene in embodiment 2 + -CrORCA3-CrG10H was transferred into Agrobacterium rhizogenes C58C1, and a single clone colony was picked for PCR verification. The results showed that the plant expression vector containing CrORCA3 and CrG10H genes had been successfully constructed in Agrobacterium rhizogenes strain C58C1.
[0043] 3.2. Agrobacterium rhizogenes mediated CrORCA3, CrG10H gene genetic transformation of camptophylla
[0044] 3.2.1. Preculture of explants
[0045] Cut the sterile hypocotyls (1-3cm) of camptophylla and inoculate them into the pre-cultivation medium (B 5 ), cultured in the dark at 25°C for 2 days. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com