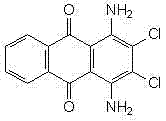
Improved method for synthesizing 1,4-diamino-2,3-dichloroanthraquinone
A technology of diaminoanthraquinone and dichloroanthraquinone is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of high recovery cost of solvent nitrobenzene or dichlorobenzene, high price of sulfuryl chloride, Problems such as small azeotrope ratio, to achieve the effects of easy recycling, low energy consumption, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Prepare raw materials according to the mass ratio of 1,4-diaminoanthraquinone leuco: solvent carbon tetrachloride: chlorine gas is 50g: 300g: 44g (1: 6: 0.88);
[0019] In a reaction vessel with a stirrer, a thermometer, a feeder, a steam distillation device and a temperature adjustment device, under stirring, dissolve 50 g of 1,4-diaminoanthraquinone leuco body in 300 g of solvent carbon tetrachloride to form a reaction liquid, then adjust the temperature of the reaction solution to 15-25°C, pass 44g of chlorine gas into the reaction solution to chlorinate the leuco 1,4-diaminoanthraquinone, the speed of passing chlorine gas is 1.5mg / s, and pass chlorine gas After 12 hours, stop chlorination, lower the temperature to 93% (mass) by liquid chromatography analysis .
Embodiment 2
[0021] Substantially the same as Example 1, the difference is: replace carbon tetrachloride with tetrachlorethylene. 60g of 1,4-diamino-2,3-dichloroanthraquinone can be obtained with a yield of about 95%. According to liquid chromatography analysis, the content of 1,4-diamino-2,3-dichloroanthraquinone is >92.5% (quality).
Embodiment 3
[0023] Prepare raw materials according to the mass ratio of 1,4-diaminoanthraquinone leuco: solvent carbon tetrachloride: chlorine gas is 50g: 400g: 40g (1: 8: 0.80);
[0024] In a reaction vessel with a stirrer, a thermometer, a feeder, a steam distillation device and a temperature controller, under stirring, 50 g of the leuco 1,4-diaminoanthraquinone is dissolved in 300 g of the solvent carbon tetrachloride to form a reaction liquid, then adjust the temperature of the reaction solution to 25-30°C, pass 40g of chlorine gas into the reaction solution to chlorinate the 1,4-diaminoanthraquinone leuco, the speed of passing chlorine gas is 1.5mg / s, and pass chlorine gas After 8 hours, stop chlorination, cool down to 95% (mass);
[0025] The above recovered filtrate was allowed to stand for stratification, and the solvent layer (carbon tetrachloride) was separated for storage and reuse.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com