Thymidine derivates as well as preparation method and applications thereof in preparing tumor developing agents as ligand
A technology of thymidine derivatives and thymidine, which is applied in the field of radiopharmaceutical chemistry and clinical nuclear medicine, can solve the problems of high price and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
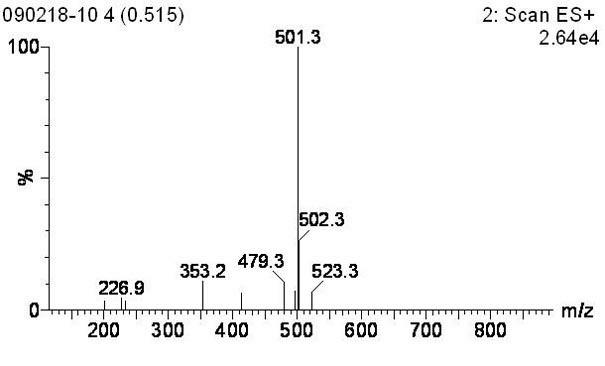
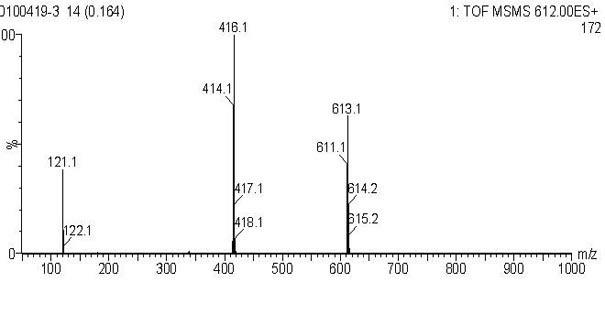
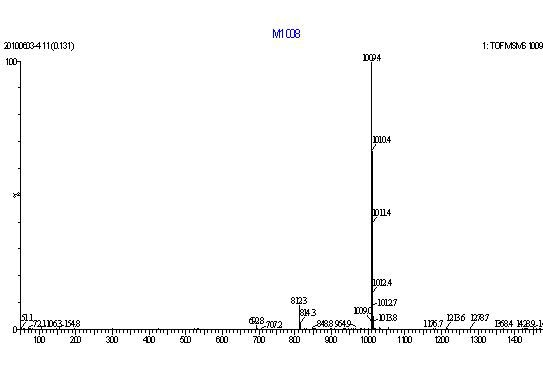
Image
Examples
Embodiment Construction
[0029] The chemical structural formula of NHT:
[0030]
[0031] The reaction equation of NHT is as follows:
[0032] The preparation method of NHT is as follows:
[0033] (1) Synthesis of 3’,5’-bis(p-methylbenzoyl)-thymidine (product 1):
[0034] Add 8g of β-thymidine (33mmol) and 10g of DMAP (4.9mmol) into a 500mL three-neck flask, add 250mL of dichloromethane, and add 10mL of p-toluyl chloride dropwise under stirring conditions. After dropping, heat to reflux , continue to stir the reaction until the reaction solution becomes clear. After the reaction is complete, the reaction solution is cooled to room temperature, washed with water and dilute acetic acid respectively, and the organic layer is separated. After the organic layer is dried and concentrated with anhydrous sodium sulfate, it is washed with an equal volume of The mixed solvent of dichloromethane and ethyl acetate was recrystallized at room temperature for 3-4 hours to obtain 13.8 g of product 1 in the for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com