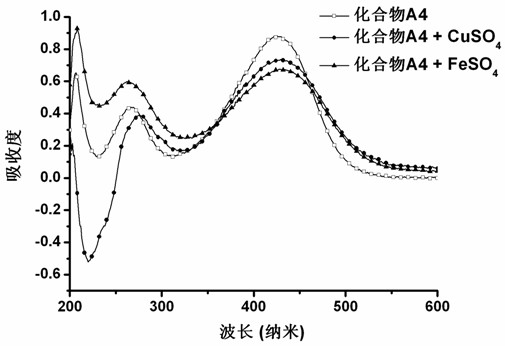
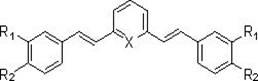
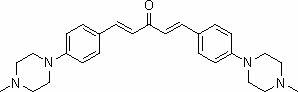
Curcumin analogue and preparation method thereof as well as application of analogue in preparation of Alzheimer disease resisting medicament
A curcumin analogue and reaction technology, which can be used in drug combinations, pharmaceutical formulations, nervous system diseases, etc., can solve problems such as inability to achieve radical cure, achieve good free radical scavenging ability, simple preparation method, and cheap raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment one: the synthesis of compound A1
[0045] At room temperature, dissolve 0.001 mol of 4-(4-methylpiperazine) benzaldehyde and 0.0005 mol of acetone in 1 mL of 95% ethanol and stir evenly, slowly drop about 0.5 mL of 10% NaOH solution into it, and stir at room temperature After about 20 minutes, a large amount of light yellow precipitate precipitated, which was suction filtered, washed with water, and dried to obtain a yellow solid powder, which was recrystallized from ethanol to obtain a light yellow powder, namely compound A1, with a yield of 85%.
[0046]
[0047] Compound A1
[0048] 1 H NMR (400 MHz, CDCl 3 ) δ 7.60 (d, J = 15.8, 2H), 7.43 (t, J = 18.7, 4H), 6.84 (dd, J = 12.0, 8.8, 6H), 3.26 (d, J = 4.6, 8H), 2.44 (d, J = 59.9, 8H), 2.37 (s, 6H); ESI-HRMS m / z : 431.2305 [M+H] + .
Embodiment 2
[0049] Embodiment two: the synthesis of compound A2
[0050] The method is the same as in Example 1, except that cyclopentanone is used instead of acetone to obtain light yellow solid A2 with a yield of 68%.
[0051]
[0052] Compound A2
[0053] 1 H NMR (400 MHz, CDCl 3 ) δ 7.45 (t, J = 9.3, 8H), 6.87 (d, J = 8.8, 4H), 3.30 – 3.23 (m, 8H), 3.00 (s, 4H), 2.54 – 2.46 (m, 8H), 2.29 (s, 6H); ESI-HRMS m / z : 457.2961 [M+H] + .
Embodiment 3
[0054] Embodiment three: the synthesis of compound A3
[0055] The method is the same as in Example 1, except that cyclohexanone is used instead of acetone to obtain light yellow solid A3 with a yield of 61%.
[0056]
[0057] Compound A3
[0058] 1 H NMR (400 MHz, CDCl 3 ) δ 7.67 (s, 2H), 7.36 (d, J = 8.7, 4H), 6.84 (d, J = 8.8, 4H), 3.28-3.18 (m, 8H), 2.85 (t, J = 5.3, 4H), 2.54-2.45 (m, 8H), 2.29 (s, 6H), 1.73 (dd, J = 11.9, 6.1, 2H).ESI-HRMS m / z : 471.3119
[0059] [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com