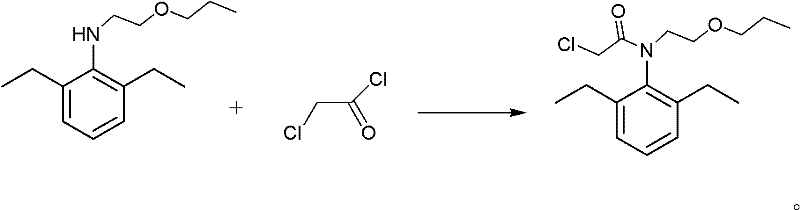
Method for synthesizing pretilachlor
A synthetic method and technology of pretilachlor, applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxyl compounds, etc., can solve the problems of low process yield, high cost of raw materials, high synthesis cost, etc., and achieve low toxicity of reagents , the number of solvents is small, and the effect of equipment corrosion is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
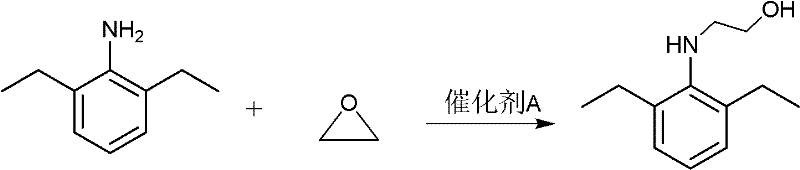
[0020] Embodiment 1: the preparation of pretilachlor.
[0021] 1) Synthesis of N-(1-methyl-2-hydroxyethyl)-2,6-diethylaniline: in a 500ml four-necked flask, equipped with electric stirring and a thermometer, add 2,6-diethylaniline ( 14.9g, 0.2mol), 0.2g of zinc chloride, 1ml of glacial acetic acid, 150ml of water, 150ml of methanol, start stirring, keep the temperature at 10-15°C, slowly introduce ethylene oxide gas, and keep it until the gas does not overflow. , and weighed at all times. After the system gained 9g in weight, stop ventilation, return to room temperature and stir for 2h. After the reaction, methanol was distilled off, separated and washed with water to obtain 23.3g of oily substance, with a yield of 95%. The target product was detected by NMR.
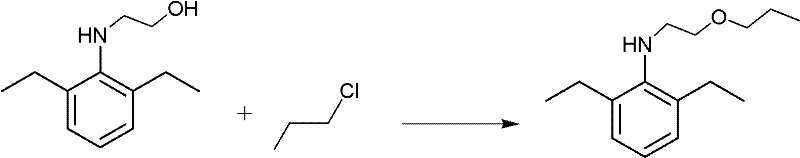
[0022] (2) Synthesis of 2-ethyl-6-methyl-N-(1'-methoxy-prop-2-yl) aniline: in a 500ml three-necked flask, an electric stirrer, a thermometer, a reflux condenser, and N -(1-methyl-2-hydroxyethyl)-2,6-diethylaniline (39...
Embodiment 2
[0025] Embodiment 2: the preparation of pretilachlor.
[0026] 1) Synthesis of N-(1-methyl-2-hydroxyethyl)-2,6-diethylaniline:
[0027] In a 500ml four-neck flask, equipped with electric stirring and a thermometer, add 2,6-diethylaniline (14.9g, 0.2mol), 0.3g of zinc acetate, 1ml of glacial acetic acid, 200ml of ethanol, start stirring, and keep the temperature at 20°C Left and right, slowly feed ethylene oxide gas, keep the gas not overflowing, and weigh it all the time. After the system has gained 9g in weight, stop the ventilation, return to room temperature and stir for 2 hours. Obtained 23.6 g of oily substance, yield: 96%. The target product was detected by NMR.
[0028] 2) Synthesis of 2-ethyl-6-methyl-N-(1'-methoxy-prop-2-yl)aniline:
[0029] In a 500ml three-necked flask equipped with an electric stirrer, a thermometer, and a reflux condenser, add N-(1-methyl-2-hydroxyethyl)-2,6-diethylaniline (39.4g, 0.2mol), normal chlorine Propane 300ml, concentration is 80% so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com