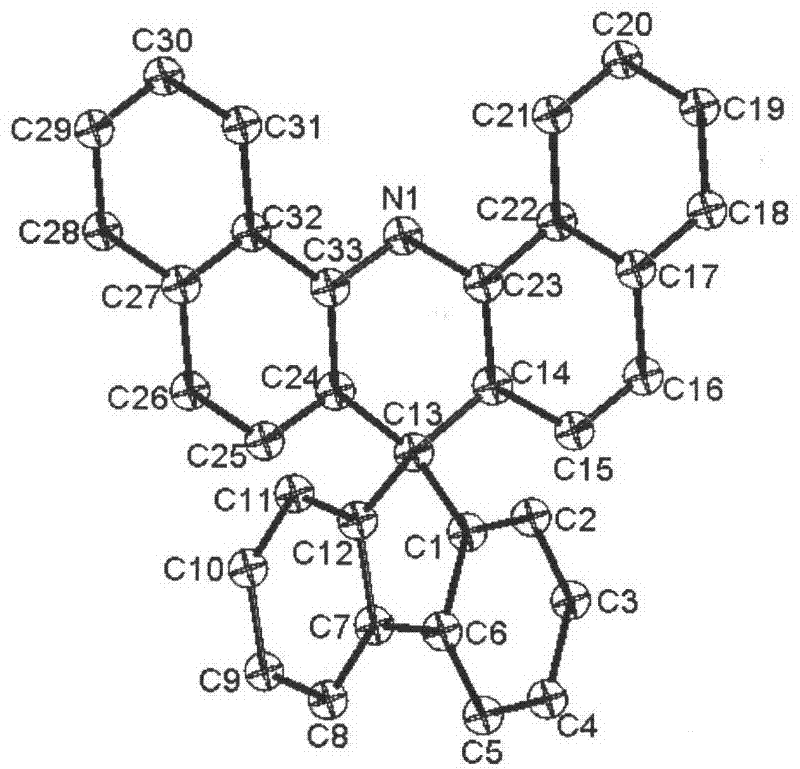
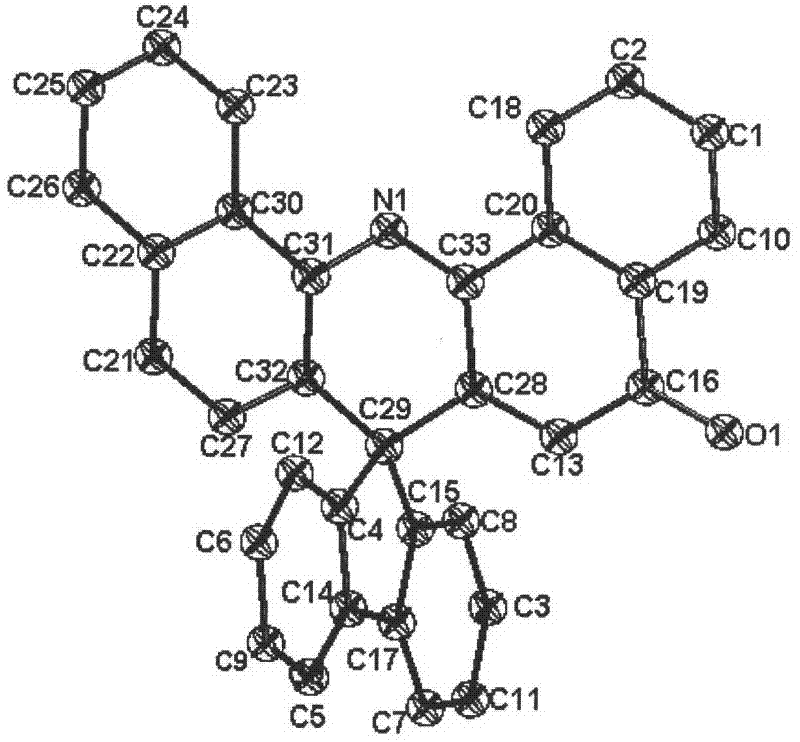
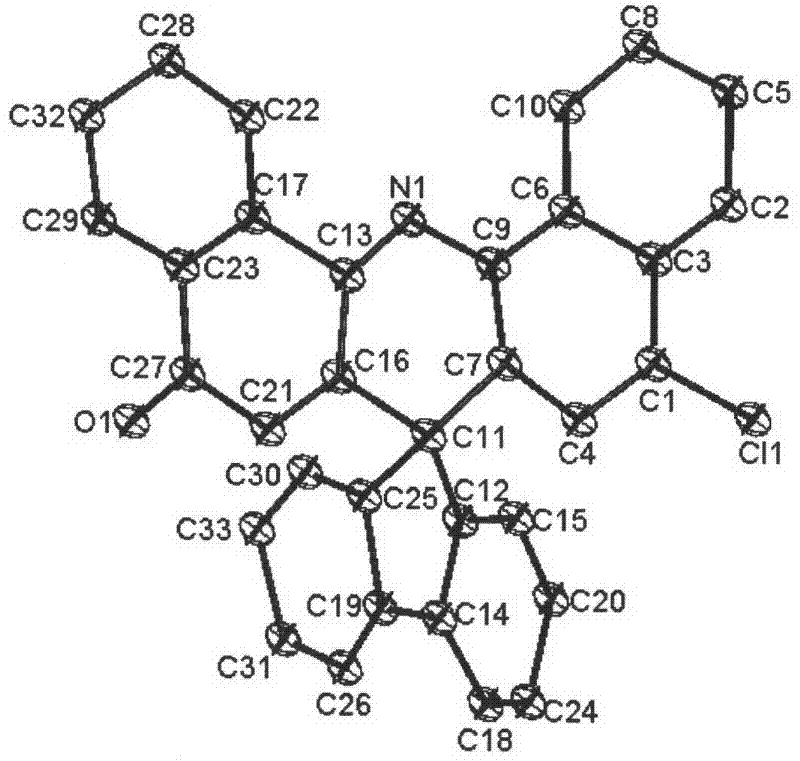
Spirofluorene bisbenzoacridine organic semiconductor material, preparation method and use method thereof
A technology of dibenzoacridine and organic semiconductors, applied in semiconductor/solid-state device manufacturing, semiconductor devices, chemical instruments and methods, etc., to achieve the effect of broad research space, application prospects and high selectivity products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、14
[0060] Embodiment 1, 14-hydrogen-spirofluorene dibenzoacridine (X 1 =X 2 =X 3 =X 4 =X 5 = Ar 1 = Ar 2 =H) synthesis:
[0061]
[0062] In a 200ml two-necked flask, add 9-fluorenone (5.0g, 1eq), 1-naphthylamine (3.97g, 1eq) and 1-naphthylamine hydrochloride (15g, 3eq), vacuumize and fill with nitrogen three times, in Under nitrogen protection, add 1,2-dichlorobenzene solvent (15ml) and heat to 180°C for 7 hours, then quickly add polyphosphoric acid (5ml), continue to react at 180°C for 20 hours, after cooling, pour into the reaction flask Add 6mol / L sodium hydroxide solution (50ml) for washing, and ultrasonically dissolve part of the solid for 20min, pour the liquid into a 1000ml separating funnel, then add dichloromethane (80ml) to the reaction flask for washing, ultrasonically for 20min, The dissolved liquid is also poured into a separatory funnel, and 300ml of common saline solution is added in the separatory funnel, extracted three times with dichloromethane (abou...
Embodiment 2
[0064] Example 2, 2'-bromo-14-hydrogen-spirofluorene dibenzoacridine (X 1 =X 2 =X 3 =X 4 =X 5 = H; Ar 1 = H, Ar 2 =Br or Ar 1 =Br, Ar 2 =H) synthesis:
[0065]
[0066] The synthetic route is basically the same as in Example 1. The starting materials and dosage of this synthesis are: fluorenone derivatives 2-bromo-9-fluorenone (5.0g, 1eq), 1-naphthylamine (2.77g, 1eq) and 1- Naphthylamine hydrochloride (10.41 g, 3 eq). 3.28 g of a light yellow solid with a purity >98% was obtained, with a yield of 33.2%. The NMR of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.18(d, J=8.4Hz, 2H), 7.83(d, J=7.6Hz, 1H), 7.77(d, J=8.0Hz, 2H), 7.74(s, 1H), 7.71(d, J=8.1Hz, 1H), 7.67(t, J=7.7Hz, 2H), 7.54(d, J=7.3Hz, 2H), 7.52-7.48(m, 1H), 7.43-7.38(m, 1H), 7.36 (s, 1H), 7.24 (t, J=6.8Hz, 2H), 7.14 (d, J=8.6Hz, 2H), 6.37 (d, J=8.6Hz, 2H). 13 C NMR(101MHz,CDCl3)δ159.59,157.43,138.42,138.04,133.31,132.49,131.01,129.83,128.94,128.87,127.92,126.69,126.32,126.12,126.00,122.39...
Embodiment 3、2
[0067] Example 3, 2', 7'-dibromo-14-hydrogen-spirofluorene dibenzoacridine (X 1 =X 2 =X 3 =X 4 =X 5 = H; Ar 1 = Ar 2 =Br) synthesis:
[0068]
[0069] The synthetic route is basically the same as that in Example 1. The starting materials and dosage of this synthesis are: derivatives of fluorenone 2,7-dibromo-9-fluorenone (5.0g, 1eq), 1-naphthylamine (2.12g, 1eq) and 1-naphthylamine hydrochloride (10.68 g, 4 eq). 2.37 g of a light yellow solid with a purity >98% was obtained, with a yield of 27%. The NMR of this compound is as follows: 1 H NMR (400MHz, CDCl 3 )δ8.18(d, J=8.4Hz, 2H), 7.79(d, J=7.8Hz, 2H), 7.76(s, 1H), 7.69(dt, J=7.0, 3.6Hz, 4H), 7.55( t, J=7.5Hz, 2H), 7.51(dd, J=8.2, 1.8Hz, 2H), 7.35(d, J=1.7Hz, 2H), 7.16(d, J=8.6Hz, 2H), 6.35( d, J=8.6Hz, 2H). 13 C NMR (101MHz, CDCl3) δ159.20, 137.16, 133.35, 132.45, 131.25, 129.92, 128.97, 126.33, 126.16, 126.10, 122.50, 122.35, 121.34, 121.32, 119.09, 1176.39, 5
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com