5-fluorouracil drug intermediate, preparation method and application thereof
A technology of fluorouracil and reaction, which is applied in the field of synthesis of 5-fluorouracil drug intermediates, can solve the problems of high toxicity and side effects, difficulty in oral absorption, low fat solubility, etc., and achieve the effect of high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
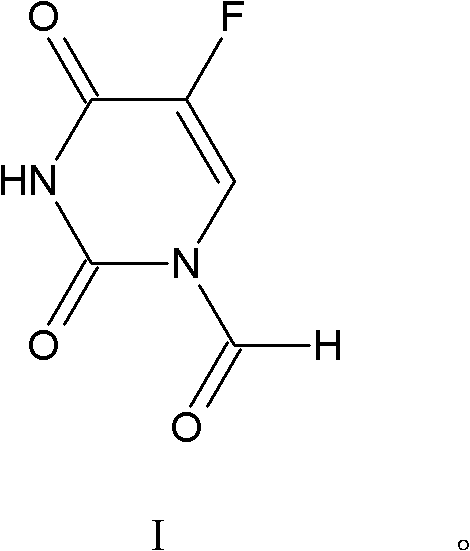
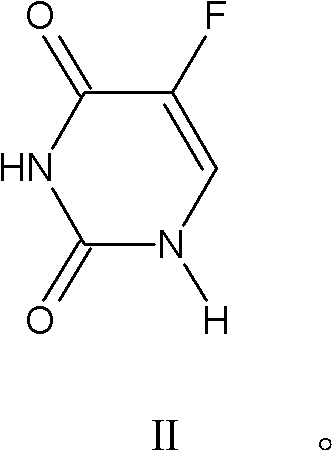
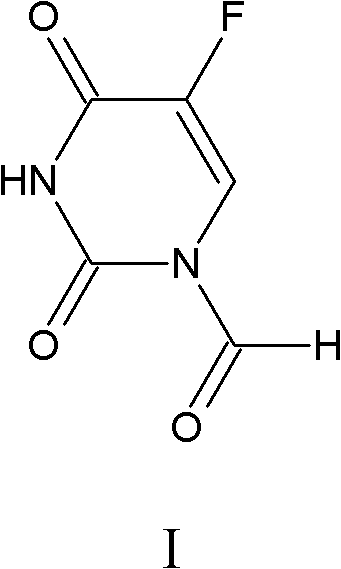
[0036] Take 1.302g of 5-fluorouracil in a 100mL three-necked flask, add 20mL of water and 5mL of 37% formaldehyde, react at 55°C, track and detect with TLC, the reaction is complete in 6 hours, distill off the water, add 30mL of acetone, 0.174g of manganese dioxide is oxidized , TLC tracking, after 4 hours of reaction, filtered, and the filtrate was evaporated to dryness to obtain 1.29 g of 5-fluorouracil-1-carbaldehyde, with a yield of 81.6%.
[0037] Spectral data of 5-fluorouracil-1-carbaldehyde: 1 H NMR (500MHz, DMSO-d 6 , TMS) δ11.20 (s, 1H, NH of 5-FU), 8.20 (s, 1H, -CHO), 7.67 (d, 1H, FC=CH, 3 J FH =6.5Hz) 13 C NMR (125MHz, DMSO-d 6 , TMS) δ, 52.0, 49.5, 36.3; IR (KBr, cm -1 )v: 3116, 1722, 1652, 1558, 1450, 1396, 1378, 1243, 1181, 679; ESI-MS m / z: 157 [M-1] - .
[0038] The NMR was completed on the AVANCE-500 NMR analyzer of Bruker, Switzerland, the mass spectrometry was completed on the DECAX-30000 LCQ DecaXP Plus of Finnigan Mass Spectrometry Company of the Un...
Embodiment 2
[0040] Take 1.307g of 5-fluorouracil in a 100mL three-necked flask, add 2mL of formic acid and 1.12g of zinc oxide, 30mL of toluene, react at 110°C, TLC tracking detection, 20h complete reaction, cool to room temperature, filter to obtain solid and filtrate, and evaporate the filtrate to dryness , to obtain part of 5-fluorouracil-1-carbaldehyde; the filtered solid was dissolved in 20mL DMF, filtered, and the filtrate was evaporated to dryness to obtain 5-fluorouracil-1-carbaldehyde, the total of the two was 0.95g, and the yield was 60.3%.
Embodiment 3
[0042]Take 1.303g of 5-fluorouracil in a 100mL three-necked flask, add 3mL of formic acid, 1.5g of silica gel powder, and 30mL of toluene, and react at 110°C. TLC tracking detection, the reaction is complete after 20 hours, cooled to room temperature, filtered to remove the silica gel, and evaporated to dryness. 0.26 g of solid 5-fluorouracil-1-carbaldehyde was obtained with a yield of 16.45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com