Beta-carboline alkaloid and application thereof to preparing antimalarial medicaments
An alkaloid, anti-malarial technology, applied in the direction of anti-infective drugs, drug combinations, resistance to vector-borne diseases, etc., can solve problems such as failure and reduction of drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
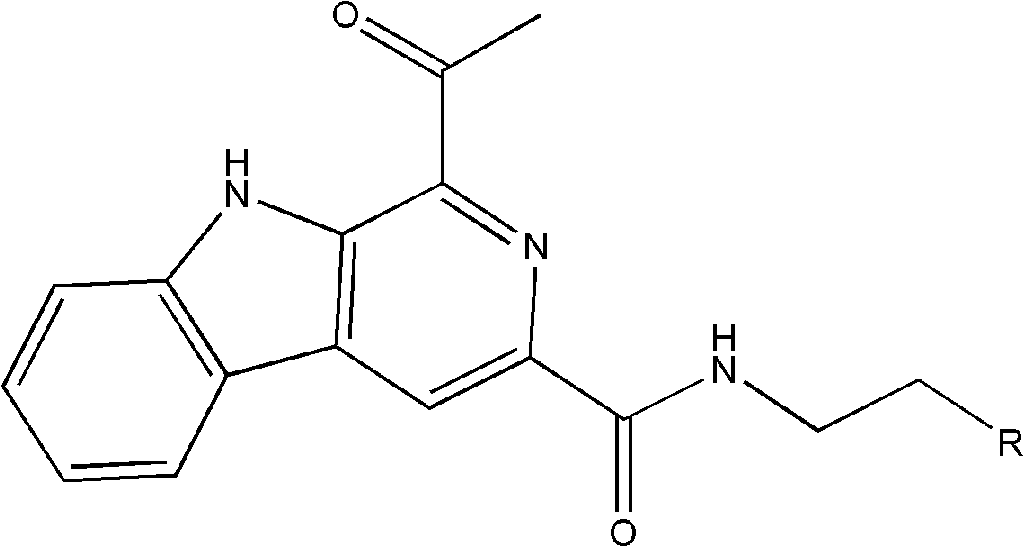
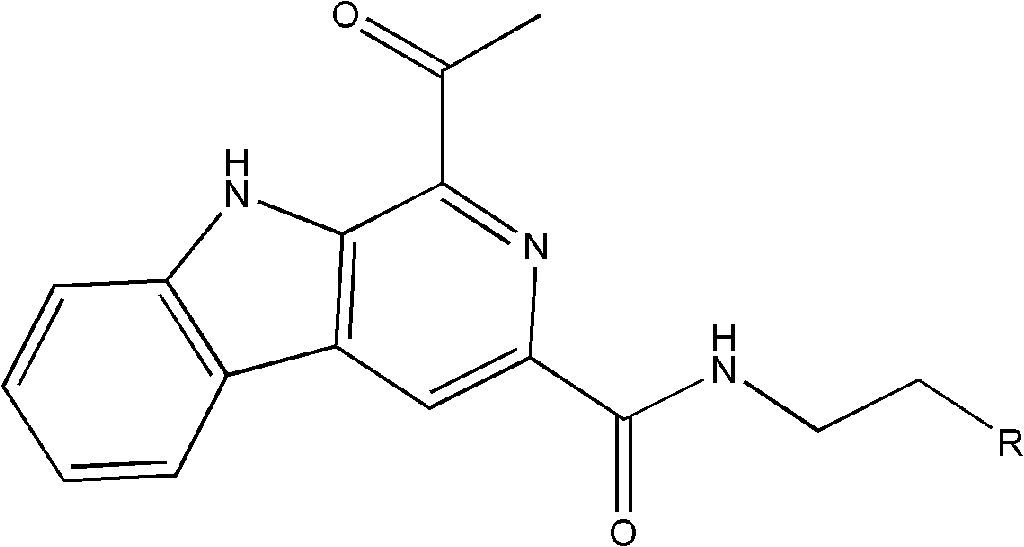
[0018] Embodiment 1: the preparation of the β-carboline alkaloid shown in formula (I):
[0019] 1. Seed culture:
[0020] (1) Preparation of seed medium: Weigh 40g of glucose, 4g of peptone, 4g of yeast extract, and 5g of sea salt, dissolve them in 2000mL of tap water, distribute them in eight 500mL Erlenmeyer flasks, and sterilize at 121°C for 25 minutes to obtain Sterilized seed medium.
[0021] (2) Cultivation of seeds: Insert the bacterial strain of marine actinomycetes (M.thermotolerans) SCSIO 00652 into the above-mentioned seed culture medium, and at a temperature of 28° C., place on a shaker at a speed of 150 rpm and cultivate for 60 hours to obtain seeds culture medium.
[0022] 2. Fermentation culture:
[0023] (1) Preparation of fermentation medium: take 100 g of soluble starch, K 2 HPO 4 10g, MgSO 4 .7H 2 O 10g, (NH 4 ) 2 SO 4 20g, CaCO 3 20g, FeSO 4 .7H 2 O 0.01g, MnCl 2 .7H 2 O 0.01g, 30g of coarse sea salt, dissolved in 10000mL of water, adjusted...
Embodiment 2
[0029] The compound isolated in Example 1 was subjected to structural analysis and testing to obtain the following physical and chemical property data:
[0030] β-carboline alkaloid 1: Pale yellow needle-like crystals; UV(PDA)λ max 220, 285, 374nm; NMR data are shown in Table 1;
[0031] (-)-ESI-MS m / z 386[M-H] - ;(-)-HR-ESI-MS m / z 386.1519[M-H] - (C 23 h 20 N 3 o 3 , theoretical value 386.1505).
[0032] β-carboline alkaloid 2: Pale yellow solid; UV(PDA)λ max 221, 285, 374nm; NMR data are shown in Table 1;
[0033] (-)-ESI-MS m / z 372[M-H] - ;(-)-HR-ESI-MS m / z 372.1350[M-H] - (C 22 h 19 N 3 o 3 , theoretical value 372.1354).
[0034] β-carboline alkaloid 3: Pale yellow solid; UV(PDA)λ max 218, 282, 373nm; NMR data are shown in Table 1;
[0035] (-)-ESI-MS m / z 356[M-H] - ;(-)-HR-ESI-MS m / z 356.1421[M-H] - (C 22 h 19 N 3 o 2 , theoretical value 356.1404).
[0036] β-carboline alkaloid 4: Pale yellow solid; UV(PDA)λ max 219, 282, 375nm; NMR data are shown...
Embodiment 3
[0054] The compounds separated and purified in Example 1: β-carboline alkaloid 1, β-carboline alkaloid 2, β-carboline alkaloid 3 and β-carboline alkaloid 4 were tested to inhibit the growth of Plasmodium.
[0055] The specific steps are as follows: culturing human Plasmodium falciparum drug-sensitive strain 3D7 and multi-drug-resistant strain Dd2 in vitro with RMPI 1640 medium and human AB serum with a volume fraction of 10%, and the hematocrit is about 2%. Plate cultured Plasmodium parasites at an initial infection rate of 0.2%, 90 μL per well. Dissolve the compound to be tested in DMSO to make a mother solution, and then dilute it with the medium. When the activity is detected, the concentration gradient is 2-fold gradient dilution, and the drug solution accounts for 10% of the volume fraction of the culture system (the concentration of DMSO is less than 0.5% of the volume fraction) , which is 10 μL. at 37°C, CO 2 Cultured in the incubator for 72h. Then add 100 μL of lysi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com