anti-muc1 antibody
An antibody and antigen technology, applied in the direction of antibodies, anti-tumor drugs, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc., can solve the problems of insufficient selectivity and unclear sugar chain specificity, etc. achieve less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267] (Example 1: Synthesis of MUC1Tn20-mer glycopeptide)
[0268] Synthesis of Example Compound 1
[0269] H-His-Gly-Val-Thr-Ser-Ala-Pro-Asp- Thr (Galβ1→3GalNAcα)-Arg-Pro-Ala-Pro-Gly-Ser-Thr-Ala-Pro-Pro-Ala-NH 2 (1)
[0270]When synthesizing the glycopeptide solid phase, use Rink Amide-PEGA resin (0.05mmol / g, 500mg, 25μmol) as the solid phase carrier. For the amino acid elongation reaction, under the condition of microwave irradiation (40W, 2450MHz, 50℃), in the DMF solution of Fmoc-amino acid derivative (75μmol), HBTU (75μmol), HOBt (75μmol), DIEA (150μmol) React for 5 minutes. For sugar chain substitution amino acid elongation reaction, use 1.5 equivalents of Fmoc-Thr(Ac6 core1)-OH: N-α-Fmoc-O-[O-(2,3,4,6-tetra-O-acetyl -β-D-galactopyranosyl)-(1→3)]-4,6-di-O-acetyl-2-acetamide-2-deoxy-α-D-galactopyranosyl}- L-threonine was reacted under the same conditions for 20 minutes. Acetylation of unreacted amino groups was carried out in 13 Mm HOBt in acetic anhydride / DIEA...
Embodiment 2
[0365] (Example 2: Production of MUCl-specific antibody)
[0366] (preparation of immunogen)
[0367] Dissolve 5 mg of 2,3-ST(DT*R)-20 (Compound No. 2 in Table 1) in 0.2 ml of distilled water, add 0.2 ml of an aqueous solution containing 860 μg of Sulfo-SMCC (manufactured by PIERCE) and 0.2 ml of 0.1 M phosphate buffer (pH 7.4), and allowed to react at room temperature for 1 hour. 200 µg of Sulfo-SMCC was added twice to the reaction solution, and the maleimidated compound 2 was purified by HPLC, followed by freeze-drying.
[0368] Dissolve 18.2 mg of BSA (manufactured by SIGMA-ALDRICH) in 0.2 ml of 0.2 M phosphate buffer (pH 7.4), add 0.2 ml of an aqueous solution containing 6 mg of Sulfo-LC-SPDP (manufactured by PIERCE), and react at room temperature 2 hours, and then react overnight at 4°C. BSA-SH in the reaction liquid was subjected to gel filtration and purification using a PD-10 column (manufactured by GE Healthcare). Gel filtration was performed on a PD-10 column equ...
Embodiment 3
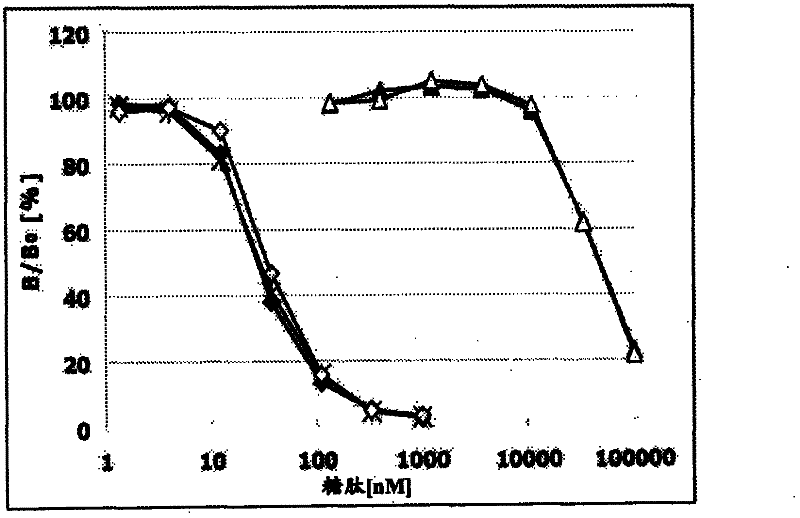
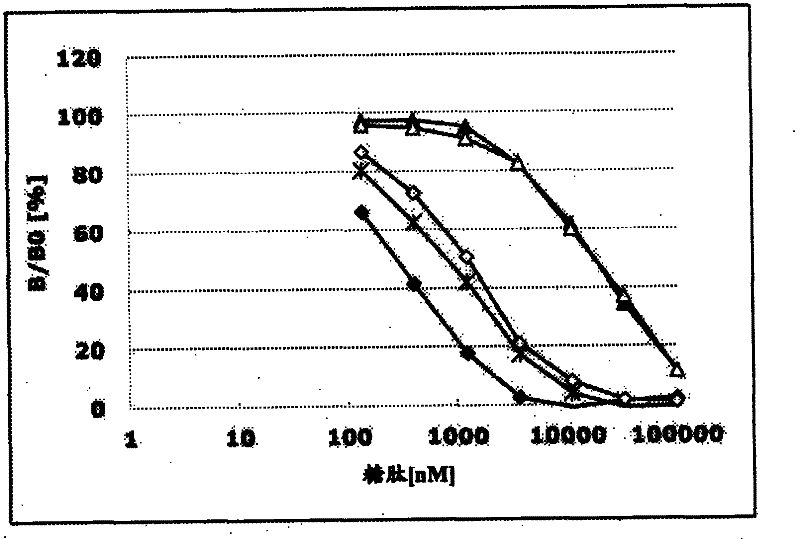
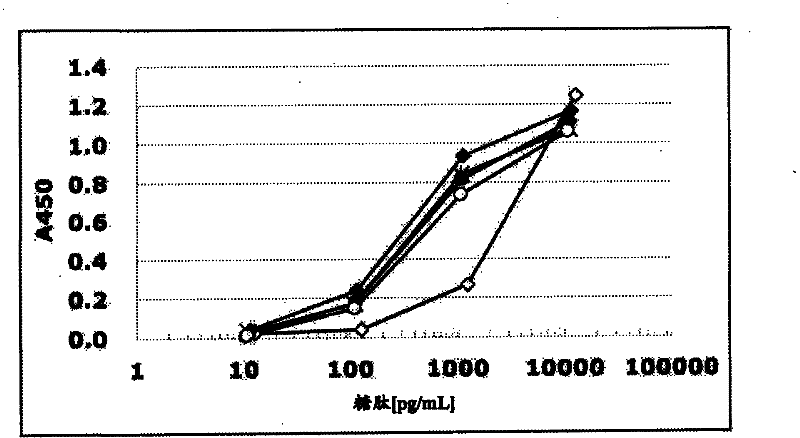
[0374] (Example 3: Determination of specificity of antibody)
[0375] (sugar chain specific)
[0376] 15 µl of MUCl antibody-containing buffer A was added to the anti-mouse IgG antibody immobilized plate, and allowed to react at room temperature for 3 hours. Next, wash each well 3 times with 90 μl washing solution, and then add streptavidin-HRP and biotin-Tn(DT*R)-100 (compound No. 21 in Table 1), and T(DT*R) respectively. R)-20 (Compound No. 1), 2,3-ST(DT*R)-20 (Compound No. 2), Tn(DT*R)-20 (Compound No. 3), STn(DT*R)-20 (Compound No. 4), 2,3ST6G(DT*R)-20 (Compound No. 6), 2,3ST6L(DT*R)-20 (Compound No. 7), 2,3-ST6SL(DT*R)-20 (Compound No. 8), C6(DT*R)-20 (Compound No. 9), ST2-6(DT*R)-20 (Compound No. 10), dST(DT*R)-20 (Compound No. 11), 2. 15 μl of buffer A of 3ST(VT*S)-20 (Compound No. 12) and 40 (Compound No. 13) were reacted at 4°C for 16 hours. Next, each well was washed 3 times with 90 μl of washing solution, then 15 μl of TMB+-Substrate-Chromogen (manufactured by D...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com