A class of novel spiroheterocyclic compounds and their use as therapeutic agents
A compound and mixture technology, applied in anti-inflammatory agents, organic chemistry, drug combination, etc., can solve the problems of low selectivity, large toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0133] Synthetic general method of embodiment 1 compound (I) and partial compound structure analysis
[0134] Dissolve 10 mmol of the corresponding substituted benzaldehyde in 10 mL of absolute ethanol, stir at room temperature for 5 min, then add the corresponding cyclopentanone, acetone or cyclohexanone, and continue stirring for 10 min, the solution remains unchanged. Sodium metal was dissolved in methanol to prepare 18% (w / v) sodium methoxide / methanol solution. Slowly add 1.5mL of the sodium methoxide solution (containing 5mmol of sodium methoxide) into the reaction solution. After stirring for 2-5 hours, a large amount of insoluble yellow matter appears. The reaction solution is detected by TLC, and the raw material 2- Black spots of bromobenzaldehyde, the product spots are clearly yellow. Stop the reaction, filter the reaction solution, wash the product with water first, then wash twice with ice ethanol and ice acetone, and dry in vacuum at 30°C overnight to obtain a ye...
Embodiment 2
[0167] Example 2 Compound (I) Kinase Activity Analysis Test in Vitro
[0168]Caliper-EZ Reader is a detection platform based on the mobility detection technology of microfluidic chip technology, which has the function of real-time kinetic detection. The microfluidic chip is to integrate the steps of sample preparation, biochemical reaction and detection on the chip, which integrates the characteristics of miniaturization, integration and automation; the detection platform applies the basic principle of capillary electrophoresis to the microfluidic environment. Enzyme assays were tested without the addition of stop reagents.
[0169] We will screen the synthesized compounds for FGFR kinase inhibitory activity on the Caliper-EZ Reader platform. The experimental steps are as follows (20μM):
[0170] 1. Prepare 1.25× kinase alkaline buffer and stop buffer.
[0171] a. 1.25× Kinase Alkaline Buffer: 62.5mM HEPES, pH 7.5, 0.001875% Brij-35, 12.5mM MgCl2, 2.5mM DTT
[0172] b. Sto...
Embodiment 3
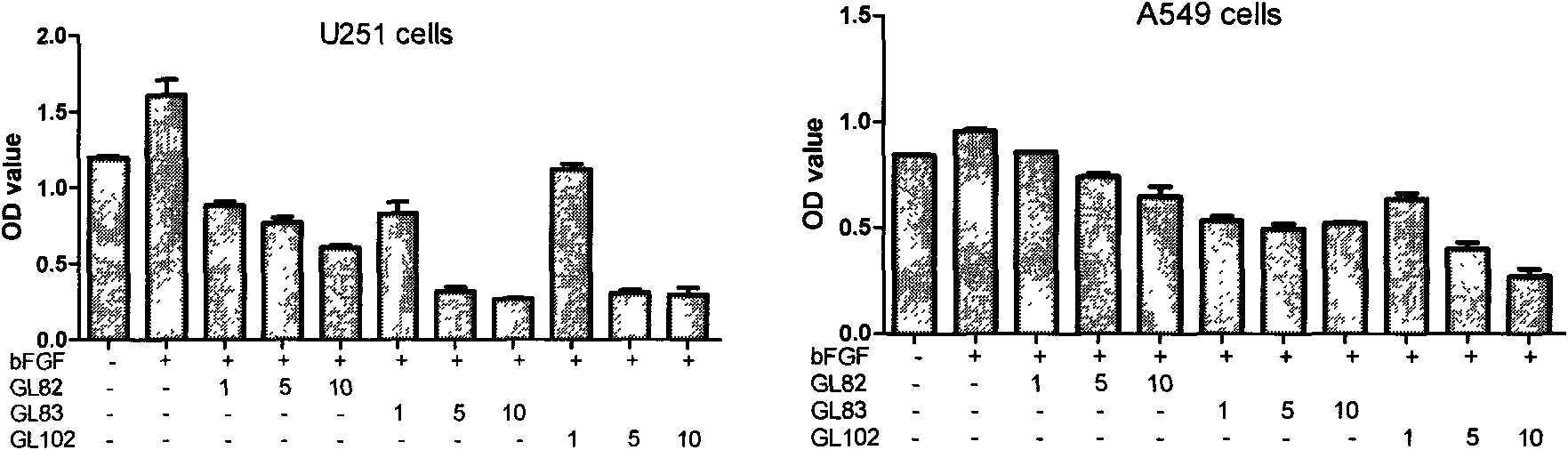
[0187] Example 3 Some compounds inhibit the proliferation of tumor cells with high FGFR1 expression induced by bFGF
[0188] The compounds with good FGFR1 tyrosine kinase inhibitory activity were selected, and their inhibitory activity on the growth of tumor cells U251 and A549 with high FGFR1 expression induced by bFGF was determined by MTT method. For the activity data, see figure 1 . bFGF was able to stimulate cell proliferation of each tumor cell. The compounds showed good activity of inhibiting bFGF-induced cell proliferation for each tumor cell, and generally showed a good dose-effect relationship, and the inhibitory effect of A549 was quite obvious.
[0189] Each cell includes: blank group, no bFGF and compound; negative group, only bFGF (20ng / mL), no compound; drug-dosed test group, bFGF (20ng / mL), plus DMSO-dissolved compound , the compound is set to three concentrations of 1 μM, 5 μM, and 10 μM. Both the blank group and the negative group were added with the same ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com