Application of recombinant Ganoderma lucidum immunoregulatory protein in the preparation of medicines for treating leukopenia caused by chemotherapy drugs
A technology for immunoregulatory proteins and leukopenia, applied in drug combinations, extracellular fluid diseases, peptide/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Obtaining of recombinant Ganoderma lucidum immunomodulatory protein
[0027] 1. Artificial synthesis of rLZ-8 gene, construction and screening of engineering bacteria
[0028] According to the genetic code preference of Pichia pastoris, on the basis of the original Ganoderma lucidum immunomodulatory protein gene sequence, the rLZ-8 gene was redesigned for whole gene synthesis, and linked with the yeast α-factor leader peptide coding sequence to form a fusion gene. Cloned into pMD18-T vector. Linearize the correctly sequenced vector, transfer it into the yeast genome, and screen methanol on the MM and MD plates to utilize the high-efficiency Mut + strain.
[0029] 2. Expression of rLZ-8 engineering bacteria
[0030] The temperature, rotation speed, pH value, liquid volume, methanol addition and other conditions of large-scale fermentation expression were tested, and the process condition optimization method for yeast engineering bacteria expressing rLZ-8 in...
Embodiment 2
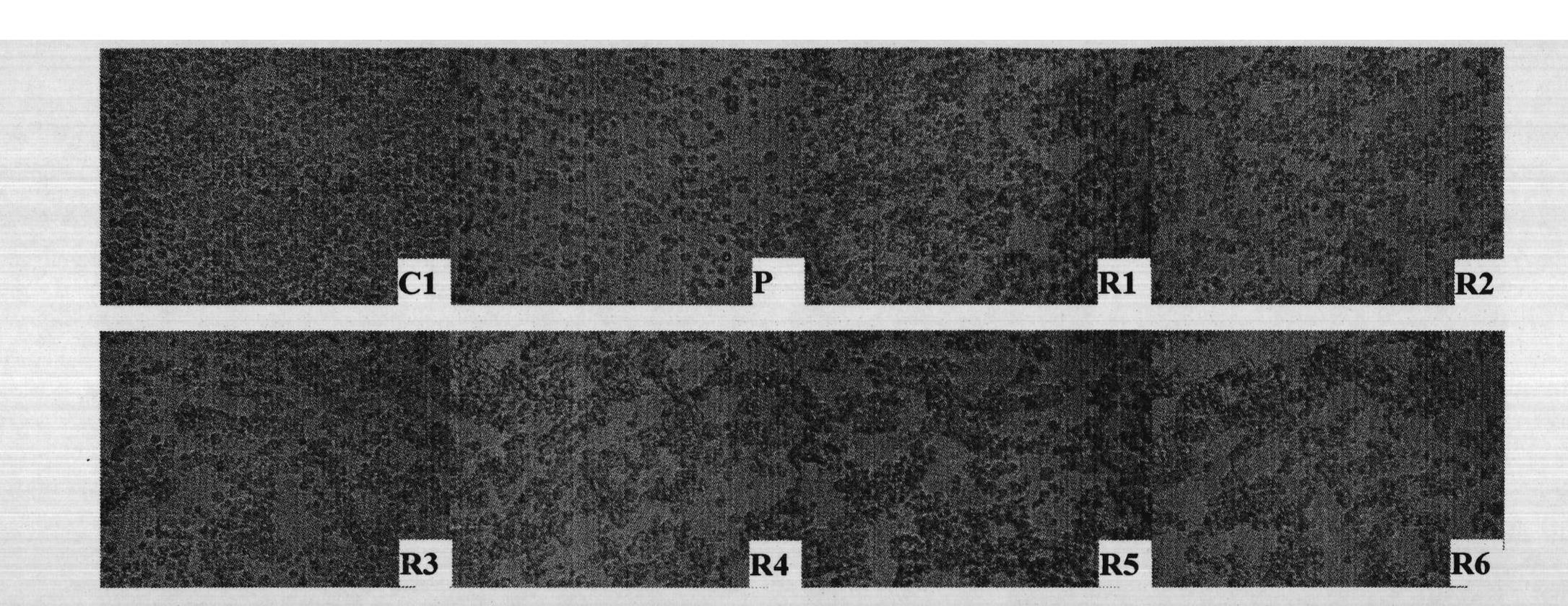
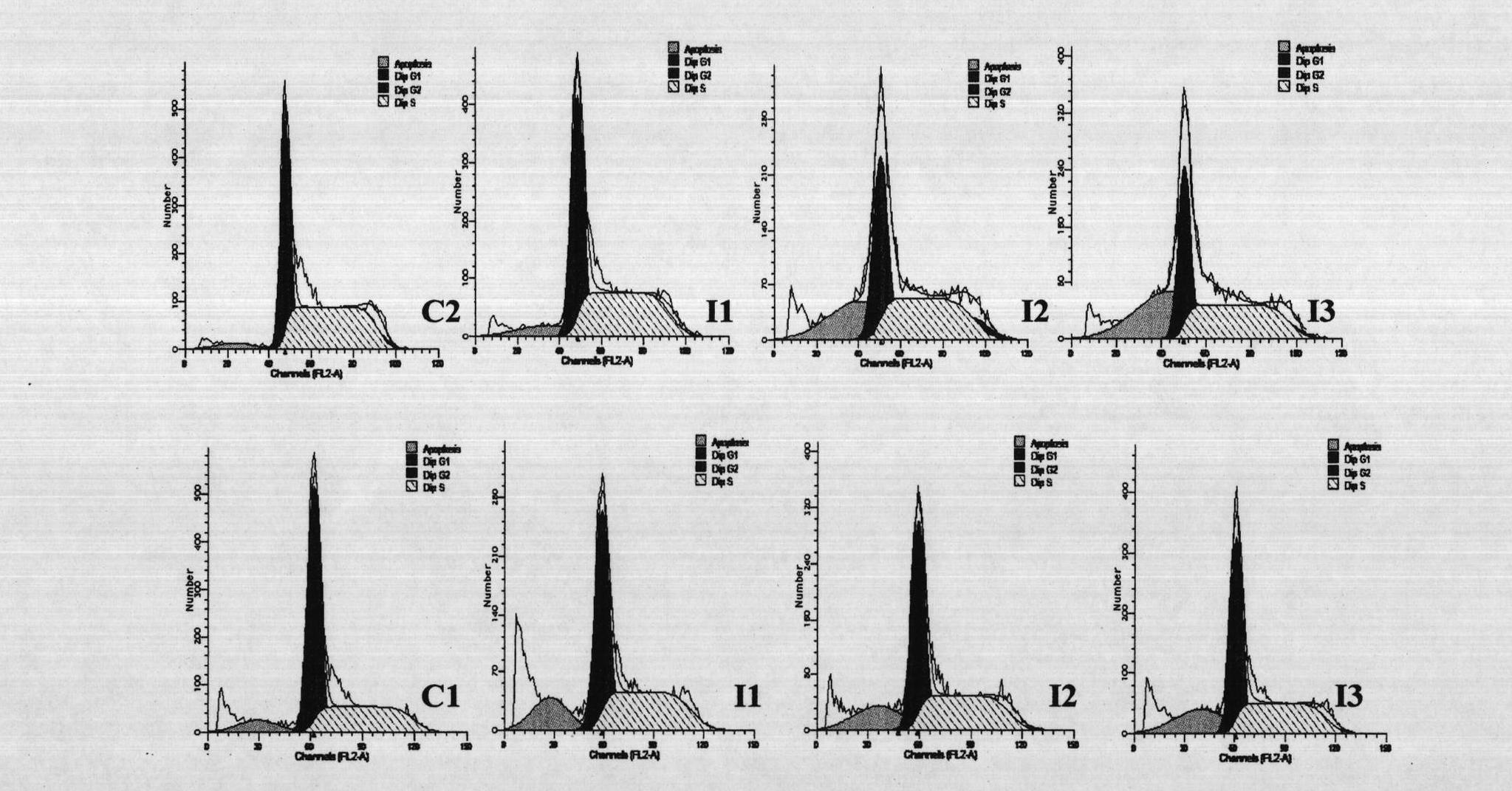
[0038] Example 2: Killing effect of rLZ-8 on human promyelocytic leukemia cell NB4
[0039] 1. Reagents
[0040] After rLZ-8 was sterilized, it was prepared into 8 concentrations with IMDM culture medium, each of which was 0.78 μg ml -1 , 1.56 μg·ml -1 , 3.125 μg·ml -1 , 6.25 μg·ml -1 , 12.5 μg·ml -1 , 25 μg·ml -1 .
[0041] 2. Experimental method
[0042] In a 96-well culture plate, add 0.1ml of NB4 tumor cells and 0.1ml of rLZ-8 to the test wells, and the concentration of rLZ-8 increases from low to high; add 0.1ml of NB4 tumor cells and culture medium to the negative control group; add arsenic trioxide As to the positive drug control group 2 o 3 ; 6 replicate holes were made in each group. Set at 37°C, 5% CO 2 48h in the incubator, add MTT15μl (5mg ml -1 ), add 100μl 0.1mol L after the termination of cell culture -1 Isopropanol hydrochloride was used to measure the OD value at 570 nm on an enzyme-linked immunosorbent detector.
[0043] 3. Experimental results
...
Embodiment 3
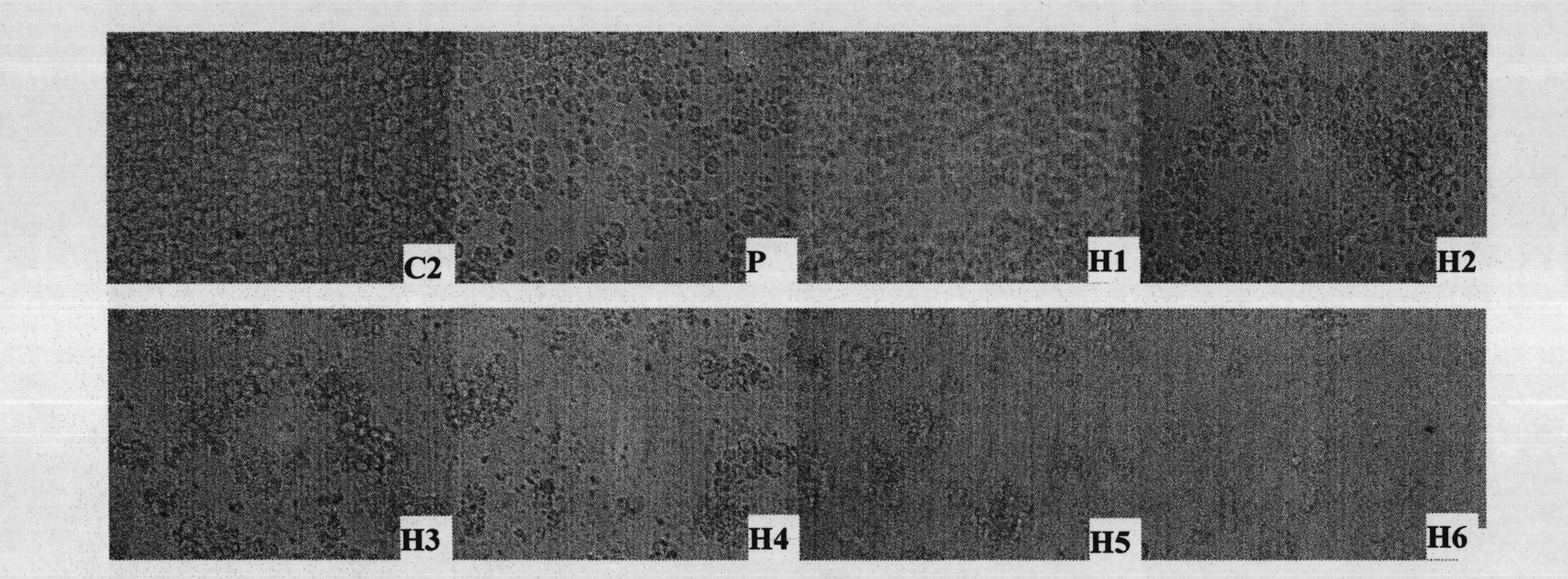
[0048] Example 3: Killing effect of rLZ-8 on human chronic myelogenous leukemia cell K562
[0049] 1. Reagents
[0050] After rLZ-8 was sterilized, it was prepared into 6 concentrations with IMDM culture medium, each of which was 3.125 μg·ml -1 , 6.25 μg·ml -1 , 12.5 μg·ml -1 , 25 μg ml -1 , 50μg·ml -1 , 100μg·ml -1 .
[0051] 2. Experimental method
[0052] In a 96-well culture plate, add 0.1ml of K562 tumor cells and 0.1ml of rLZ-8 to the test wells, and the concentration of rLZ-8 increases from low to high; add 0.1ml of K562 cells and 0.1ml of culture medium in the negative control group; positive drug arsenic trioxide; multiple holes. Set at 37°C, 5% CO 2 48h in the incubator, add MTT15μl (5mg·ml -1 ), add 100μl 0.1mol L after the termination of cell culture -1 Isopropanol hydrochloride was used to measure the OD value at 570 nm on an enzyme-linked immunosorbent detector.
[0053] 3. Experimental results
[0054] Table 2 and figure 2 As shown, the rLZ-8 drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com