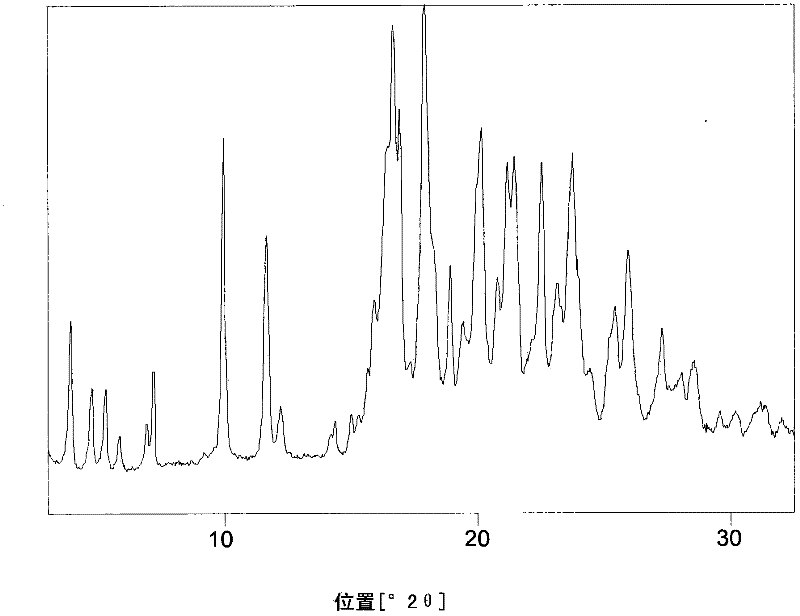
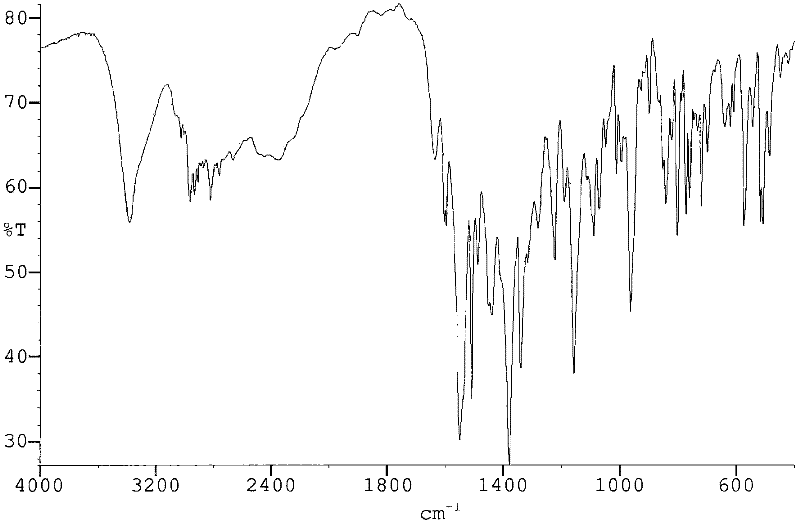
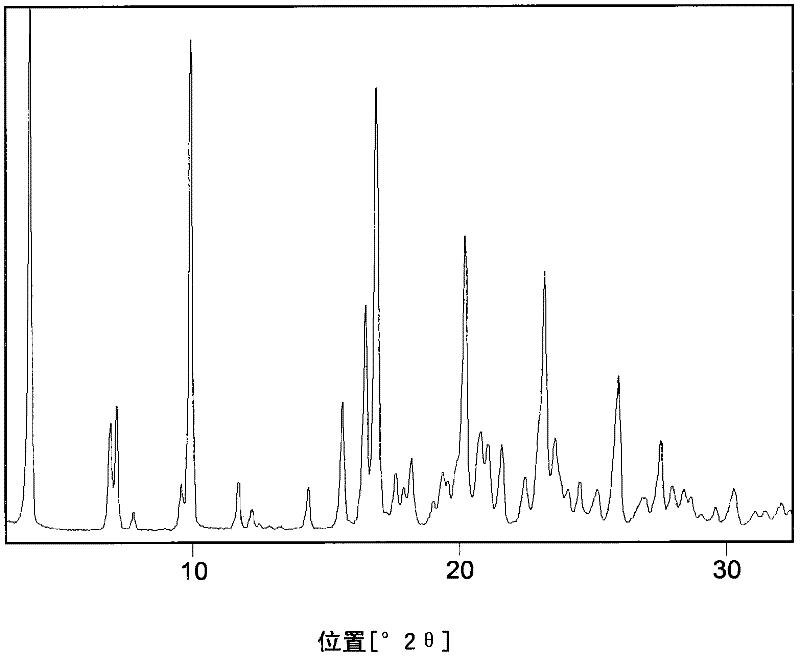
Process for preparing rosuvastatin
A technology of rosuvastatin and alkyl, applied in the field of preparation of HMG-CoA reductase inhibitors, achieving the effect of high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0239] a.) tert-butyl 2-[(4R,6S)-6-(iodomethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetate (V)
[0240] To toluene (11.5 mL) was added tert-butyl 2-[(4R,6S)-6-(hydroxymethyl)-2,2-dimethyl- 1,3-Dioxan-4-yl]acetate (1 g, 3.84 mmol), imidazole (0.523 g, 7.68 mmol) and triphenylphosphine (2.01 g, 7.78 mmol) and cooled on ice. With stirring, iodine (1.46 g, 5.80 mmol) was added in several portions. After further stirring on ice for several minutes, stirring was continued at room temperature for 2.5 h. The white precipitate formed was removed by filtration and the filtrate was evaporated to dryness to afford 1.295 g (91%) of the title compound as a yellow oil. IR: 2978, 1727, 1380, 1368, 1261, 1203, 1158, 951, 844.
[0241] b.) tert-butyl 2-{(4R, 6S)-6-[(triphenylphosphonium)-methyl]-2,2-dimethyl-1,3-dioxan-4-yl]ethyl Ester iodide (IV)
[0242] In a nitrogen atmosphere at reflux temperature, tert-butyl 2-[(4R,6S)-6-(iodomethyl)-2,2-dimethyl-1,3-dioxan-4-yl]ethane Ester (1.61 g, 4.35...
Embodiment 1-2
[0244] a.) tert-butyl 2-{(4R,6S)-6-[(tert-butyldiphenylsilyloxy)methyl]-2,2-dimethyl-1,3-dioxane- 4-yl]acetate
[0245] Tert-butyl 2-[(4R,6S)-6-(hydroxymethyl)-2,2-dimethyl-1,3-dioxan-4-yl]acetate (20mmol, 5.20 g) was dissolved in dichloromethane (12 mL), imidazole (1.852 g) was added, and the mixture was cooled on ice. Tert-butyldiphenylchlorosilane (6.24 mL) was added with stirring. After stirring on ice for another 15 min, stirring was continued at room temperature for 20 min. The formed precipitate was removed by filtration, and the filtrate was successively washed with 5% aqueous HCl, 5% NaHCO 3 Aqueous solution and water wash. Anhydrous Na for organic phase 2 SO 4 Drying, filtration and evaporation of the solvent gave 9.689 g (97%) of the title compound as an oily residue. IR: 2931, 2858, 1730, 1368, 1152, 1113, 702. 1 H-NMR (CDCl 3 ): δ=1.05(s, 9H, tBu), 1.20(dd, 1H, J=24.31, 11.70Hz), 1.35(s, 3H), 1.43(s, 3H), 1.45(s, 9H), 1.69( ddd, 1H, J=12.75, 2.48Hz), 2.3...
Embodiment 1-3
[0262] (E)-7-{4-(4-fluorophenyl)-6-isopropyl-2-[methyl(methylsulfonyl)amino]-pyrimidin-5-yl}-(3R,5S)-3 , 5-dihydroxy-hept-6-enoic acid sodium salt (rosuvastatin-Na, IA-Na)
[0263] Rosuvastatin (200 mmol) was dissolved in ethanol (1347 mL) generally according to the procedure disclosed in EP 0 521 471 . 0.1M NaOH (1672 mL) was added slowly with stirring and cooling. After the addition of NaOH was complete, stirring was continued for 1 h at room temperature. The solvent was evaporated under reduced pressure and the oily residue was treated with ether (400 mL). Precipitated crystals of rosuvastatin sodium salt were filtered off. Optionally, if no crystals formed, the ether was distilled off and crystals gradually formed from the resulting foamed oil. IR: 3421, 2968, 1605, 1546, 1510, 1381, 1155, 963, 834, 775, 519.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com