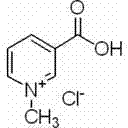
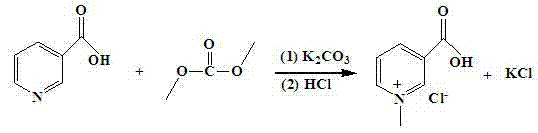Process for synthesizing trigonelline hydrochloride from dimethyl carbonate and nicotinic acid
A technology of trigonelline hydrochloride and dimethyl carbonate, which is applied in the field of synthesis technology of trigonelline hydrochloride, can solve problems such as restricting the application of trigonelline hydrochloride, increasing scientific research and production process risks, etc., and achieves high conversion rate and environmental friendliness. , the effect of simple process steps and conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 49.24 g of nicotinic acid (0.40 mol) to a 100 ml three-necked round-bottom flask with a reflux condenser and stirring, use 67.41 ml (0.80 mol) of dimethyl carbonate as the solvent and stir until dissolved, add 55.28 g of K 2 CO 3 (0.40 mol) and continue to stir for 10 minutes, heated in a water bath to control the temperature at 50°C, and monitored the reaction by thin layer chromatography (TLC) during the reaction. After the reaction, the potassium carbonate K was removed by centrifugation. 2 CO 3 , the pressure is 150-260 Pa, the liquid phase temperature is 60-85 ° C, and the unreacted solvent dimethyl carbonate is removed by concentration under reduced pressure. The residual solid was dissolved with 4 mol / L hydrochloric acid and the pH was adjusted, and the solvent was removed under reduced pressure. Add an appropriate amount of anhydrous methanol to the residual solid and shake thoroughly, and then centrifuge to remove insoluble matter after standing still. ...
Embodiment 2
[0018] Add 49.24 g of nicotinic acid (0.40 mol) to a 100 ml three-necked round-bottom flask with a reflux condenser and stirring, use 33.6 ml (0.88 mol) of dimethyl carbonate as the solvent and stir until dissolved, add 55.28 g of K 2 CO 3 (0.40 mol) and continue to stir for 10 minutes, heated in a water bath to control the temperature at 50°C, and monitored the reaction by thin-layer chromatography during the reaction. After the reaction, the potassium carbonate K was removed by centrifugation. 2 CO 3 , the pressure is 150-260 Pa, the liquid phase temperature is 60-85 ° C, and the unreacted solvent dimethyl carbonate is removed by concentration under reduced pressure. The residual solid was dissolved with 4 mol / L hydrochloric acid and the pH was adjusted, and the solvent was removed under reduced pressure. Add an appropriate amount of anhydrous methanol to the residual solid and shake thoroughly, and then centrifuge to remove insoluble matter after standing still. The fi...
Embodiment 3
[0020] Add 49.24 g of nicotinic acid (0.40 mol) to a 100 ml three-necked round-bottomed flask with a reflux condenser and stirring, stir in 33.6 ml (0.88 mol) dimethyl carbonate until dissolved, add 66.34 g of K 2 CO 3 (0.48 mol) and continue to stir for 10 minutes, heated in a water bath to control the temperature at 50°C, and monitored the reaction by thin-layer chromatography during the reaction. After the reaction, the potassium carbonate K was removed by centrifugation. 2 CO 3 , the pressure is 150-260 Pa, the liquid phase temperature is 60-85 ° C, and the unreacted solvent dimethyl carbonate is removed by concentration under reduced pressure. The residual solid was dissolved with 4 mol / L hydrochloric acid and the pH was adjusted, and the solvent was removed under reduced pressure. Add an appropriate amount of anhydrous methanol to the residual solid and shake thoroughly, and then centrifuge to remove insoluble matter after standing still. The filtrate was concentrat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com