Method for preparing palladium nitrate
A technology of palladium nitrate and concentrated nitric acid, applied in chemical instruments and methods, ruthenium/rhodium/palladium/osmium/iridium/platinum compounds, inorganic chemistry, etc., can solve problems such as slow dissolution speed, consumption of concentrated nitric acid, etc., and achieve dissolution The effect of increased speed, low production cost and simple preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
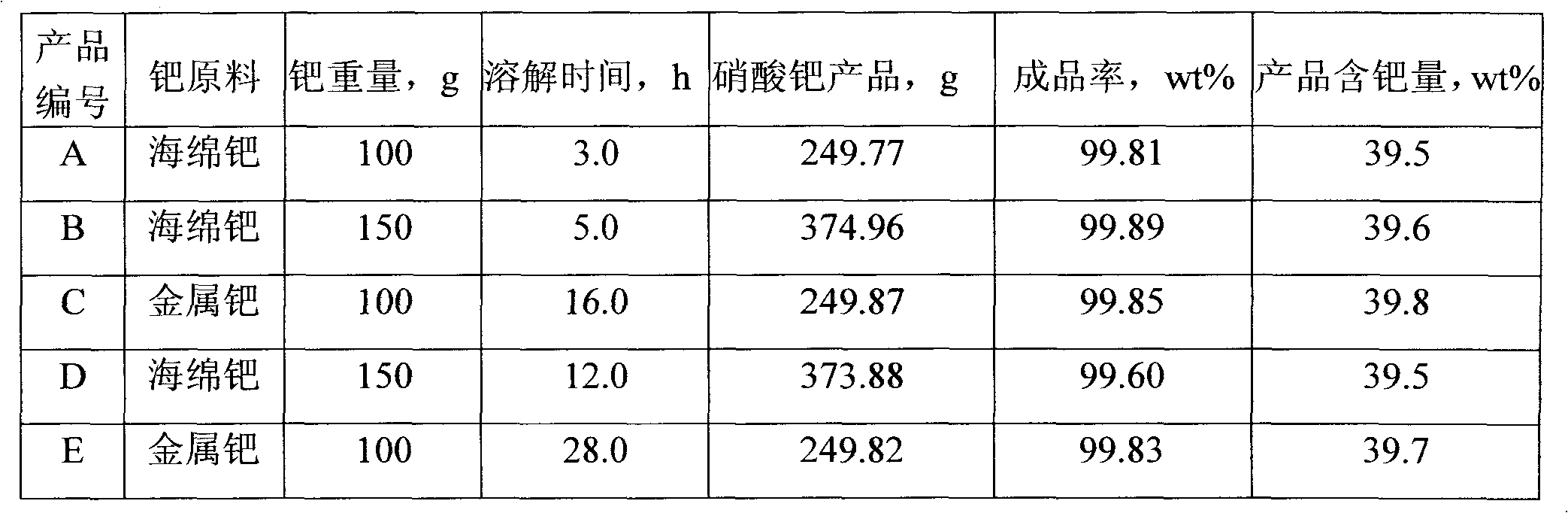
[0018] Get 100 grams of sponge palladium powder with a purity > 99.95%, put it into a 2-liter beaker, add 300 grams of concentrated nitric acid with a weight concentration of 65% and 250 grams of hydrogen peroxide with a weight concentration of 30% slowly in two batches, each batch is Add concentrated nitric acid slowly first, and then add hydrogen peroxide immediately after the addition. The amount of the two added each time is 1 / 2 of the total amount. Heat the solution on an electric heater to 45°C. After dissolving, cool to room temperature and filter, then heat The solution was evaporated and concentrated to a slightly boiling state until a layer of crystal film appeared on the liquid surface, cooled and crystallized, and the obtained brown crystals were washed with deionized water at 6°C until there was no free nitric acid. Then put it into a vacuum oven with a vacuum degree of 0.05MPa, and dry it at 50°C for 8 hours, and the product [Pd(NO 3 ) 2 · 2H 2 O] After passin...
Embodiment 2
[0020] Get 150 grams of sponge palladium powder with a purity > 99.95%, put it into a 2-liter beaker, add 600 grams of concentrated nitric acid with a weight concentration of 65% and 300 grams of hydrogen peroxide with a weight concentration of 30% slowly in three batches, each batch is Add concentrated nitric acid slowly first, then add hydrogen peroxide immediately after the addition, the amount of the two added each time is one-third of the total, heat the solution on an electric heater to 50°C, after dissolving, cool to room temperature and filter, heat The solution was evaporated and concentrated to a slightly boiling state until a layer of crystal film appeared on the liquid surface, cooled and crystallized, and the obtained brown crystals were washed with 8°C deionized water until there was no free nitric acid. Then put it into a vacuum oven with a vacuum degree of 0.05MPa, and dry it at 50°C for 8 hours, and the product [Pd(NO 3 ) 2 · 2H 2 O] After passing the test,...
Embodiment 3
[0022] Get 100 grams of metal palladium blocks with a purity > 99.95%, put them into a 2-liter beaker, add 700 grams of concentrated nitric acid with a weight concentration of 68% and 600 grams of hydrogen peroxide with a weight concentration of 28% slowly in four batches, each batch is Add concentrated nitric acid slowly first, and then add hydrogen peroxide immediately after the addition. The amount of the two added each time is 1 / 4 of the total amount. Heat the solution on an electric heater to 55°C. After dissolving, cool to room temperature and filter, then heat The solution was evaporated to a slightly boiling state until a layer of crystal film appeared on the liquid surface, cooled and crystallized, and the obtained brown crystals were washed with deionized water at 5°C until no free nitric acid was present. Then put it into a vacuum oven with a vacuum degree of 0.06MPa, and dry it at 55°C for 7 hours, and the product [Pd(NO 3 ) 2 · 2H 2 O] After passing the test, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com