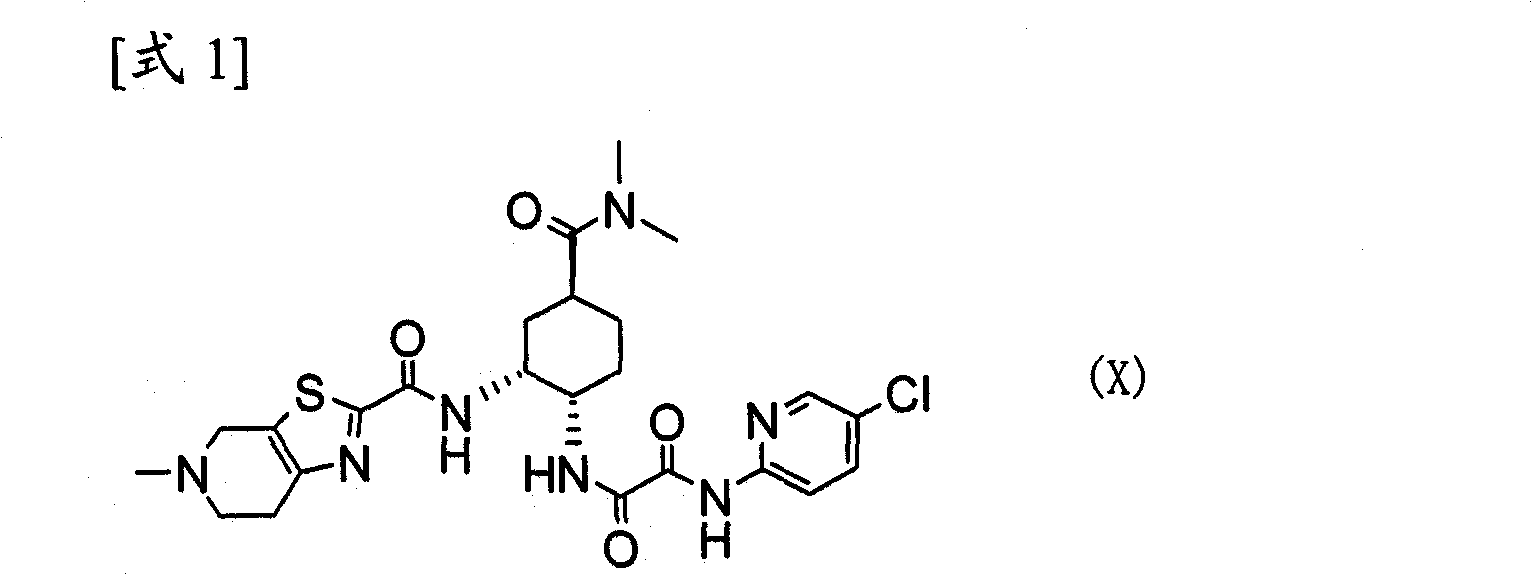
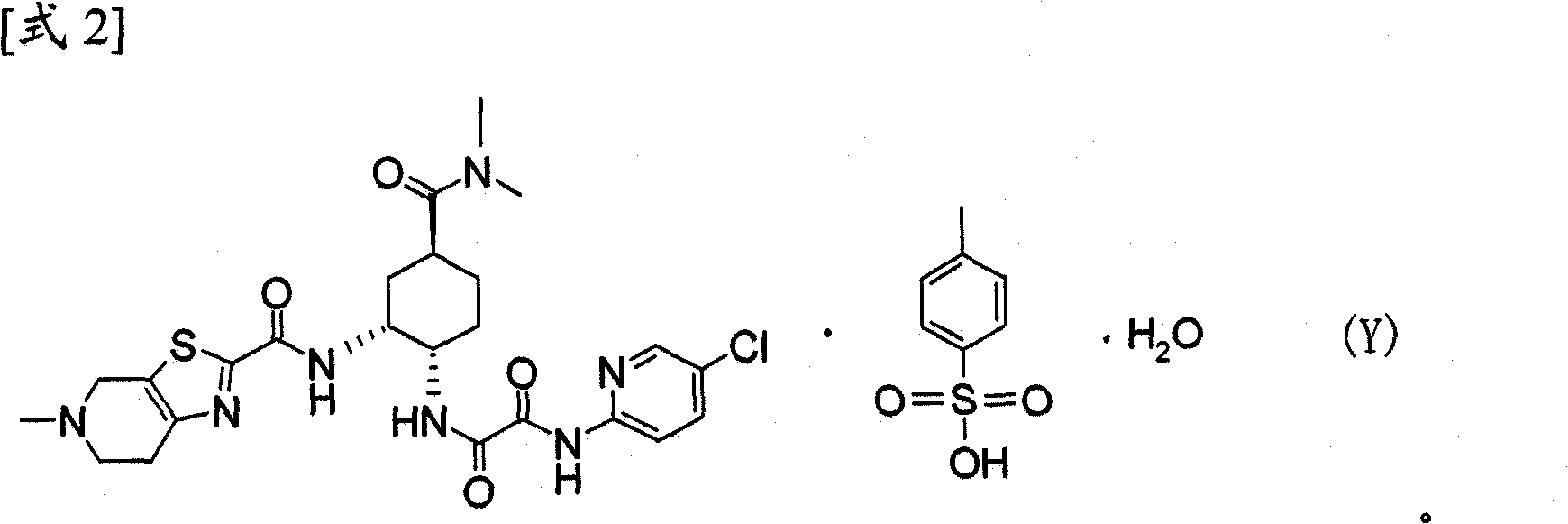
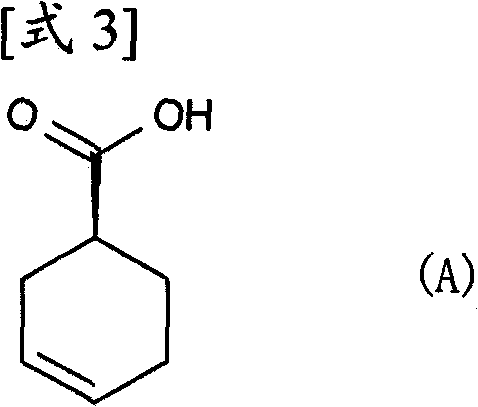
Process for producing optically active carboxylic acid
A production method and technology of water ethyl acetate, applied in the production field of optically active carboxylic acid, can solve the problems such as expensive D-pantolactone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] (Example 1) (R)-α-phenethylamine salt of (S)-3-cyclohexene-1-carboxylic acid
[0133] 3-Cyclohexene-1-carboxylic acid (1.0 kg) was dissolved in 4.8% aqueous acetone (7.5 L). To this solution was gradually added a solution of (R)-α-phenethylamine (624.3 g) dissolved in 4.8% aqueous acetone (500 ml) at 50°C, and the mixture was stirred at this temperature for 4 hours. The suspension was cooled to 35°C and stirred at this temperature for 16 hours, then further stirred at 10°C for 3 hours. The suspension was filtered under reduced pressure to obtain 837.1 g of the title compound as white crystals. Its optical purity is 63%de. Subsequently, 4.8% aqueous acetone (5.6 L) was added to the obtained salt (700 g), and the mixture was stirred under heating to reflux for 5 hours, at 30° C. for 13 hours, and then under cooling with ice for 3 hours. The suspension was filtered under reduced pressure to obtain 519.4 g of the title compound as white crystals. Its optical purity is 8...
Embodiment 2
[0138] (Example 2) (R)-α-phenethylamine salt of (S)-3-cyclohexene-1-carboxylic acid
[0139] 3-Cyclohexene-1-carboxylic acid (30 g) was dissolved in 3% aqueous ethyl acetate (150 ml). To this solution was gradually added a solution of (R)-α-phenethylamine (23.0 g) dissolved in 3% aqueous ethyl acetate (30 ml) at 55°C, and the mixture was stirred at this temperature for 6 hours. The suspension was stirred at 25°C for 5 hours and further at -10°C for 2.5 hours. The suspension was filtered under reduced pressure to obtain 32.9 g of the title compound as white crystals. Its optical purity is 49%de. To the obtained salt (32.7 g) was then added 3% aqueous ethyl acetate (196 ml), and the mixture was stirred at 55°C for 3 hours, then at 25°C for 5 hours, and further at -10°C for 2.5 hours. The suspension was filtered under reduced pressure to obtain 24.7 g of the title compound as white crystals. Its optical purity is 78%. To the obtained salt (24.6 g) was further added 3% aqueou...
Embodiment 3
[0146] (Example 3) (S)-3-cyclohexene-1-carboxylic acid
[0147] To (R)-α-phenethylamine salt of (S)-3-cyclohexene-1-carboxylic acid (1.0 g, 97% de) was added methyl tert-butyl ether (20 ml) and 1N hydrochloric acid solution until the The pH of the solution becomes 1. The mixture was stirred at room temperature for 1 hour. The organic layer was dried over anhydrous magnesium sulfate, and then the solvent was distilled off to obtain 504 mg of the title compound as a colorless oil.
[0148] 1H-NMR (CDCl3) δ: 1.64-1.75 (1H, m), 1.99-2.20 (3H, m), 2.24-2.30 (2H, m), 2.56-2.63 (1H, m), 5.63-5.70 (2H, m)
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com