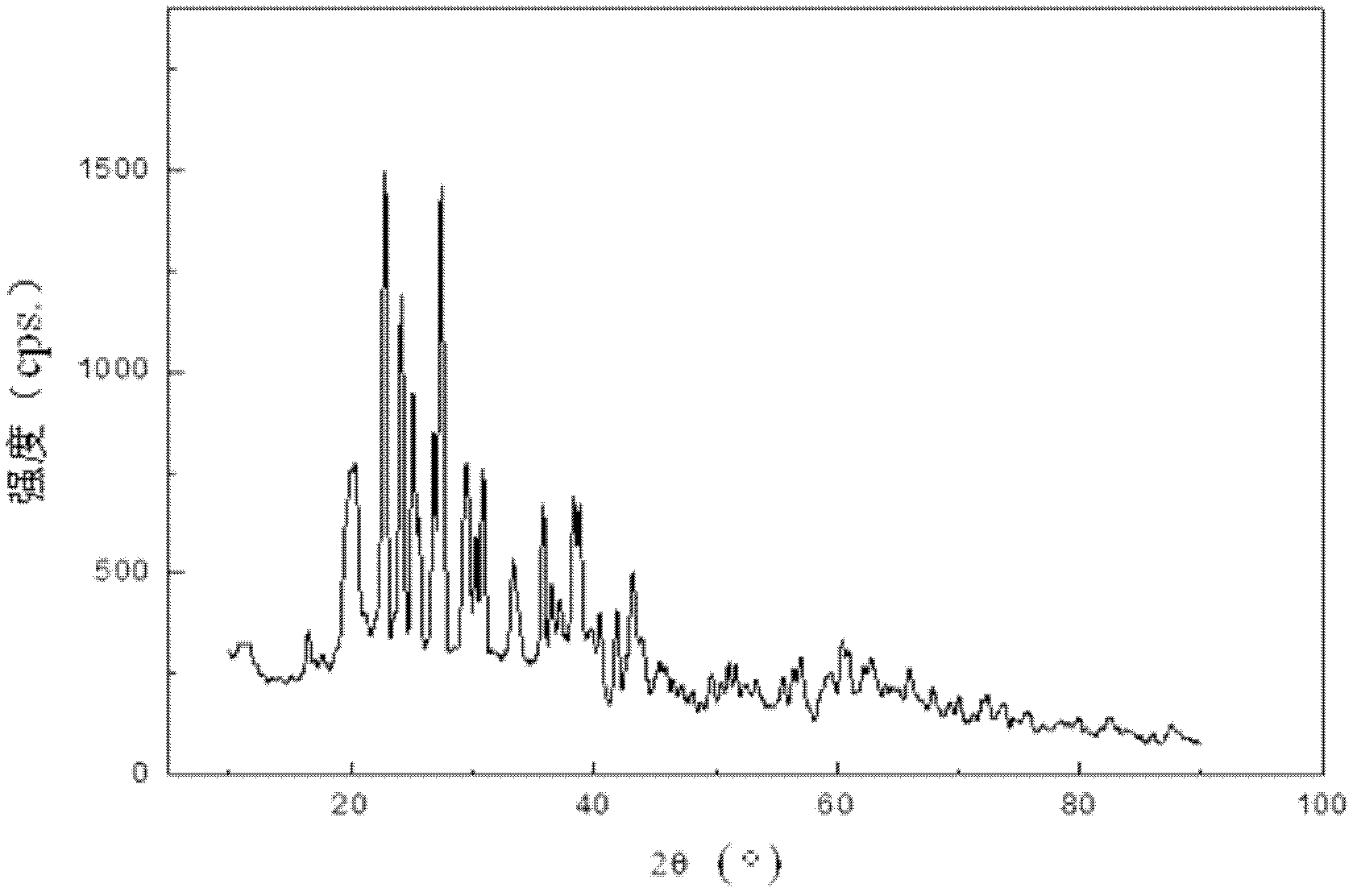
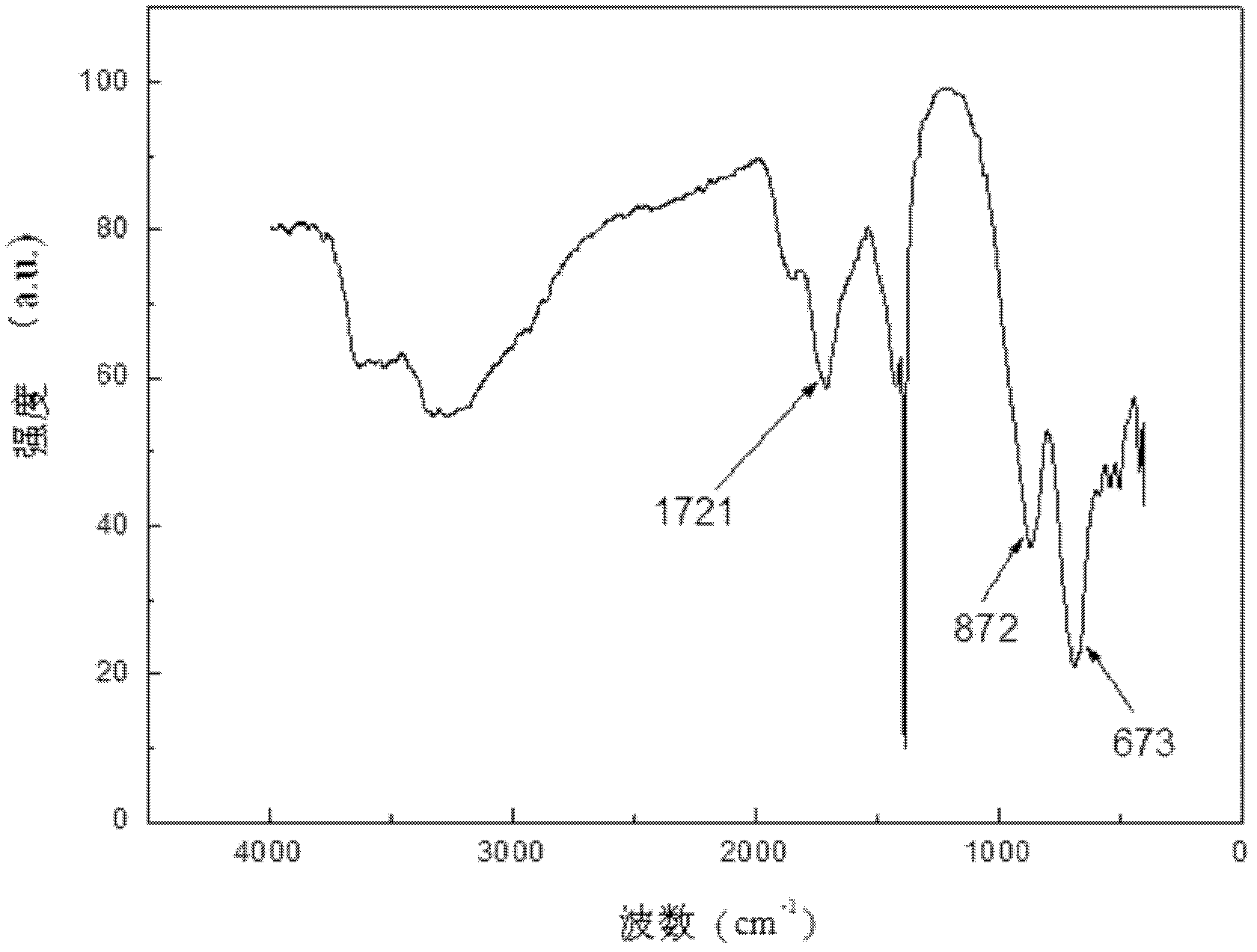
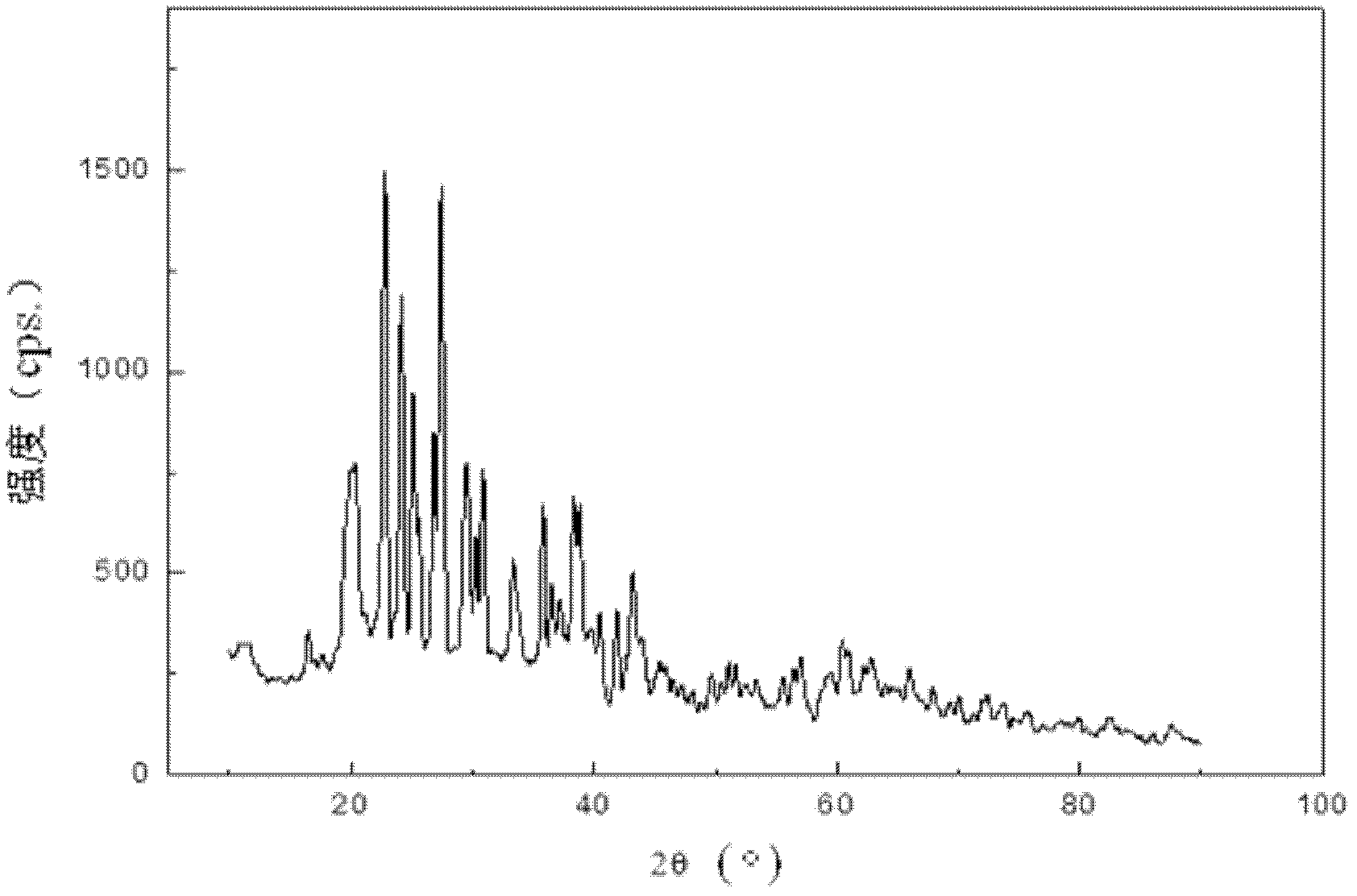
Method for directionally controlling the reaction of LiH and AlCl3 with catalyst to synthesize A1H3
A directional control and catalyst technology, applied in chemical instruments and methods, inorganic chemistry, metal hydride, etc., can solve the problems of AlH3 instability and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0013] Specific implementation mode 1: The catalyst of this embodiment controls LiH and AlCl in a directional manner 3 Reaction to synthesize AlH 3 The method is carried out according to the following steps: one, press anhydrous AlCl under anhydrous anaerobic condition 3 The mass ratio to tertiary amine is 1:6.5~65, anhydrous AlCl 3 The mass ratio of anhydrous aromatic hydrocarbon solvent is 1:4.5~45 and weigh anhydrous AlCl 3 1. Tertiary amine and anhydrous aromatic hydrocarbon solvent and mix uniformly to obtain solution A; 2. Under anhydrous and oxygen-free conditions, press LiH and anhydrous AlCl in step 1 3 The molar ratio of LiH and anhydrous aromatic hydrocarbon solvent is 1: 3~8, and the mass ratio of LiH and anhydrous aromatic hydrocarbon solvent is 1: 35~350. Weigh LiH and anhydrous aromatic hydrocarbon solvent and mix uniformly to obtain solution B; Add the solution B obtained in step 2 to the solution A obtained in step 1 under the condition of stirring, heat to...
specific Embodiment approach 2
[0015] Embodiment 2: This embodiment differs from Embodiment 1 in that the aromatic hydrocarbon solvent in step 1 is toluene, benzene, xylene or tetrahydrofuran. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0016] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: anhydrous AlCl in step one 3 The mass ratio to tertiary amine is 1:8~60, anhydrous AlCl 3 The mass ratio to the anhydrous aromatic hydrocarbon solvent is 1:5-40. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com