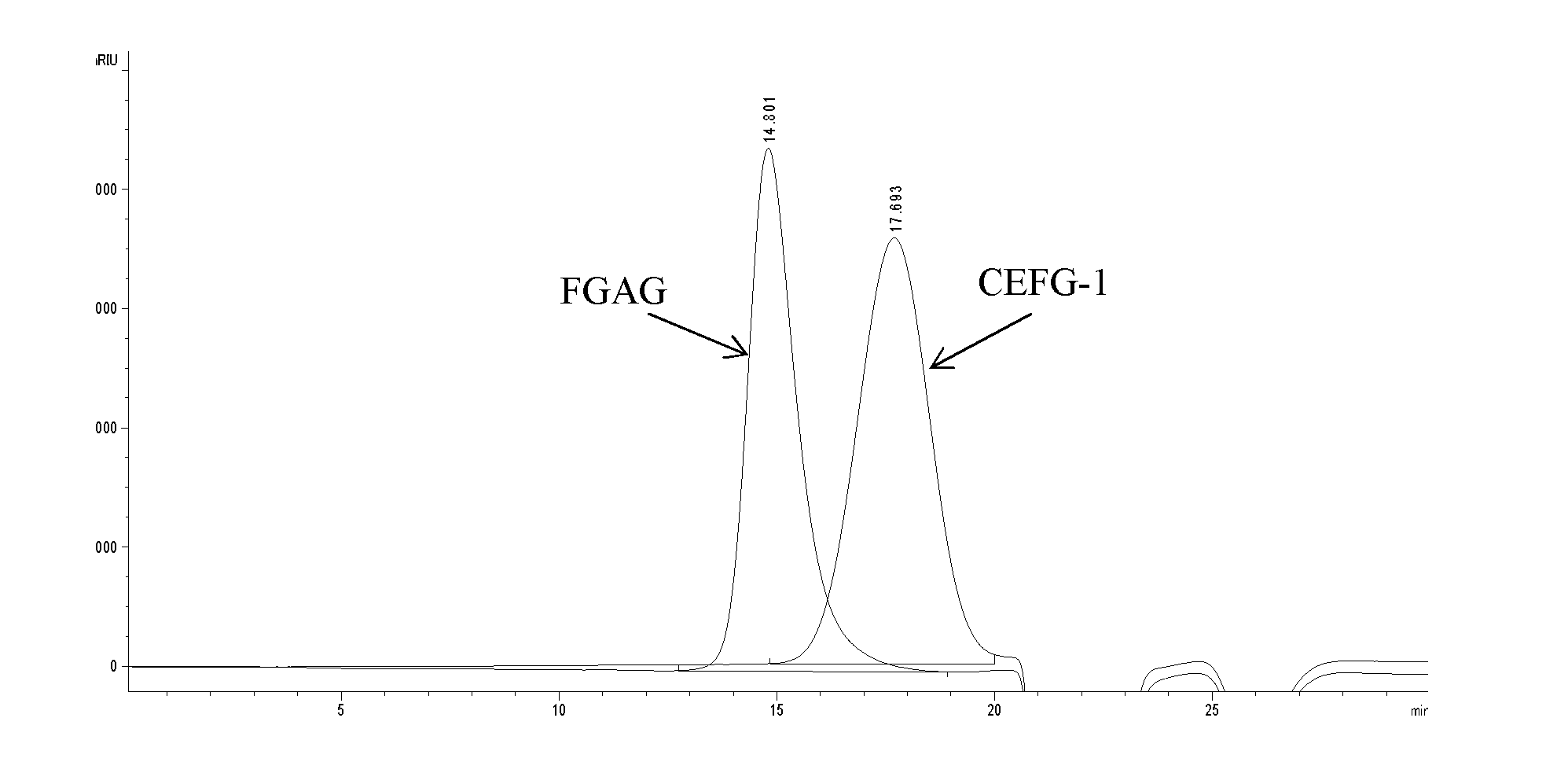
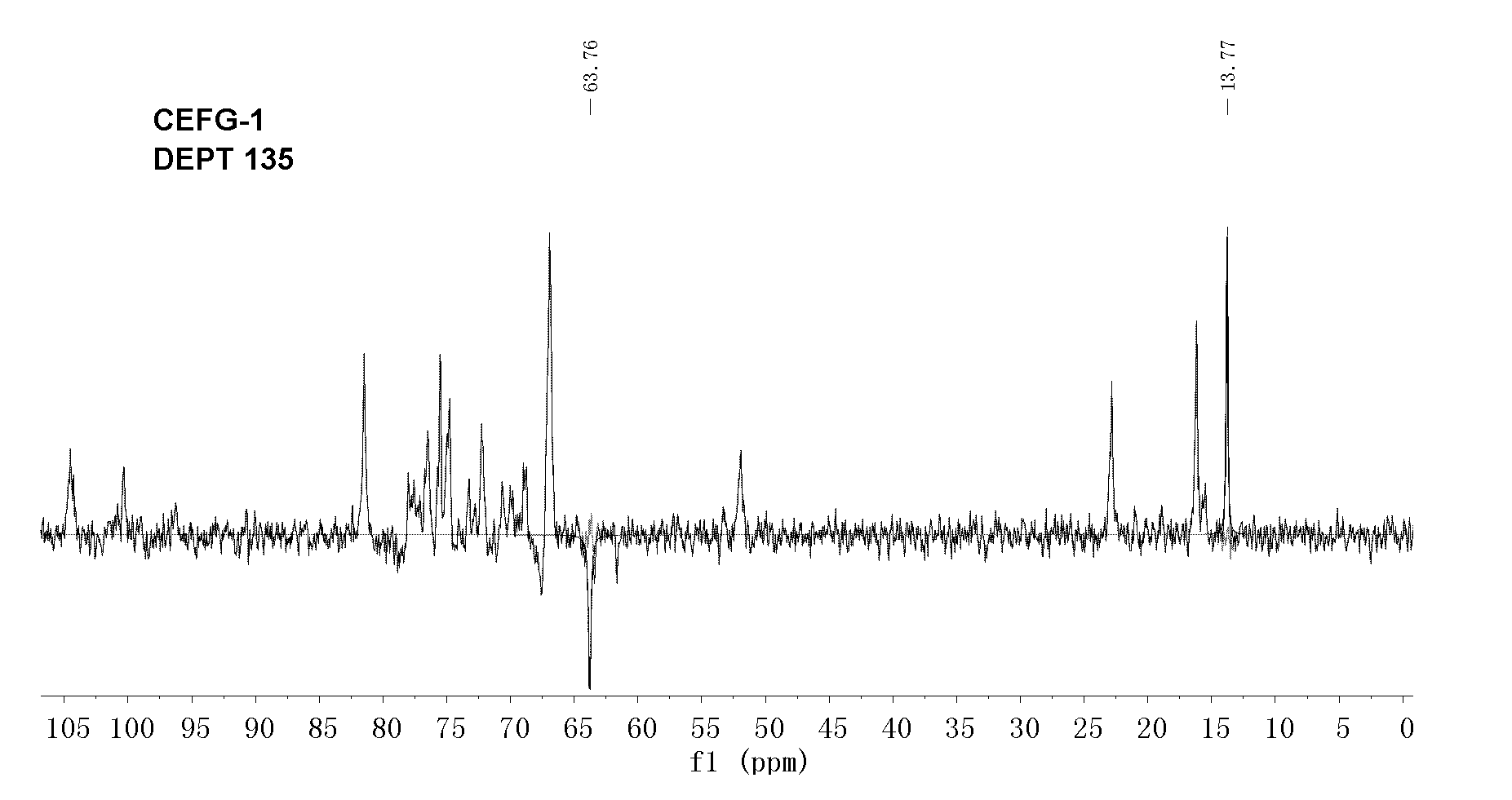
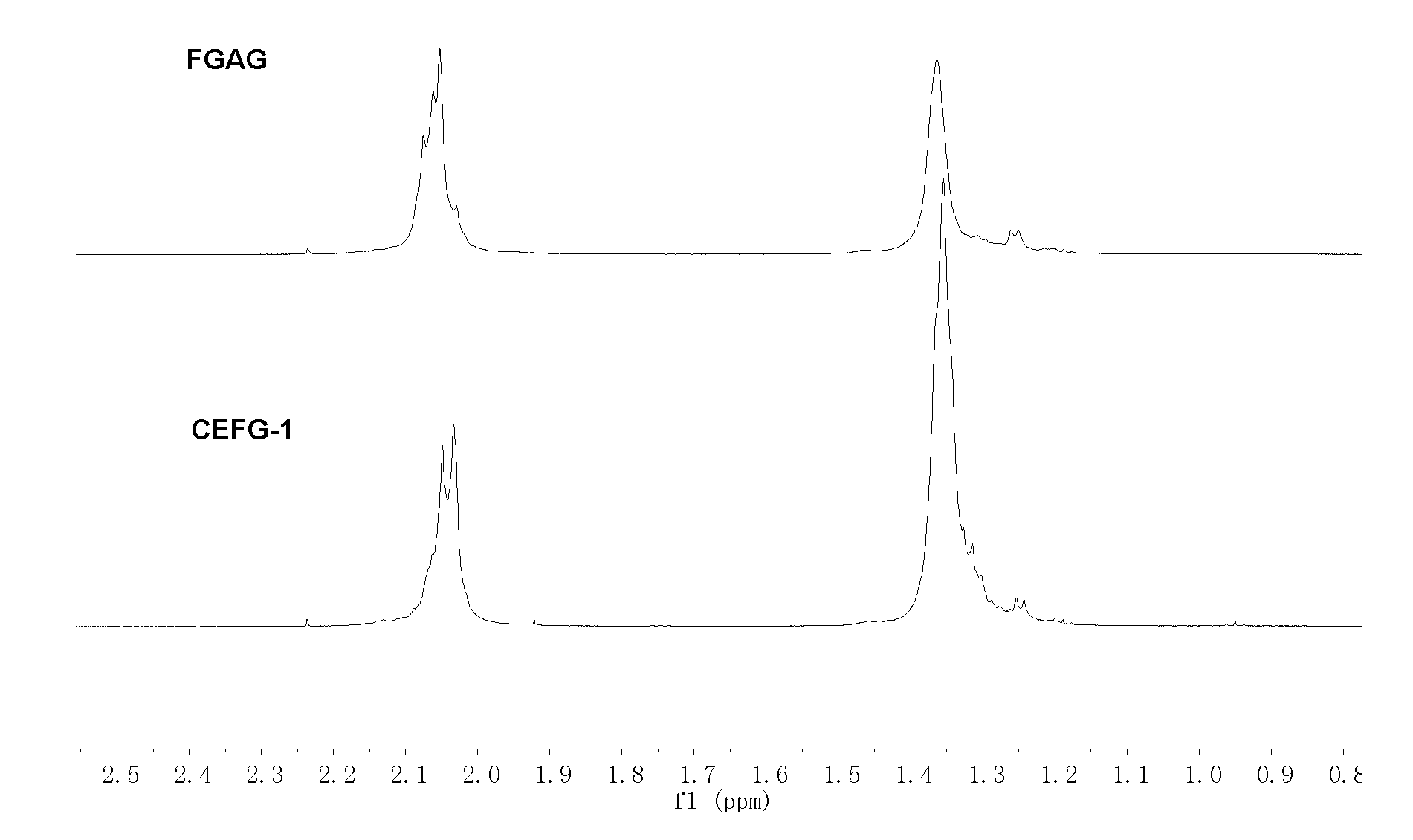Fucosylated glycosaminoglycan derivative and preparation method thereof
A technology for saccharifying glycosaminoglycan carboxylate and saccharifying glycosamine, which is applied in the field of fucosylated glycosaminoglycan carboxylate and its preparation, and can solve the problems of slow onset, large individual differences and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] [Example 1] Preparation of FGAG carboxyethyl esterification product
[0054] 1.1 Materials
[0055] Plum blossom ginseng (Thelenata ananas Jaeger), commercially available, eviscerated and dried body wall;
[0056] h 2 o 2 ,CH 3 COONa·3H 2 O, NaCl, NaOH, Cu(CH 3 COO) 2 ·H 2 O, tetrabutylammonium hydroxide, ethyl bromide, N,N-dimethylformamide, sodium hydroxide, sodium chloride and ethanol were commercially available analytical reagents.
[0057] 1.2 Method
[0058] (1) Extraction and preparation of fucosylated glycosaminoglycans (FGAG): Take the dried body wall of Echinodermata ginseng, and prepare FGAG according to the literature method (J Biol Chem, 1991, 266(21): 13530-6). The yield 0.75%, purity 98% (HPGPC, area normalization method), weight average molecular weight (Mw), 65,960.
[0059] (2) Preparation of low-molecular-weight fucosylated glycosaminoglycan (FGAG): 5.0 g of FGAG obtained in step (1) was prepared according to the method of patent CN201110114...
Embodiment 2
[0070] [Example 2] Preparation of FGAG-carboxyallyl esterification product
[0071] 2.1 Materials
[0072] Haitian melon (Acaudiina molpadioides Sepmper) is commercially available, its viscera are removed and its body wall is dried; allyl bromide is a commercially available reagent of analytical grade, and the rest of the reagents are the same as in [Example 1].
[0073] 2.2 Method
[0074] (1) Preparation of FGAG quaternary ammonium salt
[0075] According to the preparation of steps (1) to (3) in the method of [Example 1], 253.1 mg of ammonium salt was obtained. (2) Preparation of FGAG-carboxyallyl esterification product
[0076] Take 253.1 mg of the quaternary ammonium salt obtained in step (1) and place it in a reaction test tube, add 3 ml of DMF to dissolve it, add 120 μl of the reactant propylene bromide, and put it under N 2 Protected, protected from light, reacted at 30°C for 24h under stirring (450r / min). After the reaction, add 3ml of 0.5M NaCl and 15ml of dehyd...
Embodiment 3
[0077] [Example 3] Preparation of FGAG-carboxy n-butyl esterification product
[0078] 3.1 Materials
[0079] Sea cucumber (Apostichopus japonicus Selenka, 1867), commercially available, eviscerated and dried body wall; n-butane bromide and tributylamine are commercially available reagents of analytical grade, and the rest of the reagents are the same as in [Example 1].
[0080] 3.2 Method
[0081] (1) Preparation of FGAG quaternary ammonium salt
[0082] According to the method of steps (1) to (3) of [Example 1], it was prepared using tributylamine to obtain 166.6 mg of ammonium salt.
[0083] (2) Preparation of FGAG-carboxy n-butyl ester product
[0084] Take 166.6 mg of the quaternary ammonium salt obtained in step (1) and place it in a reaction test tube, add 2 ml of DMSO to dissolve it, add 150 μl of n-butane bromide as the reactant, and put it under N 2 Protected and reacted at 30°C for 20h. After the reaction, add 2ml of 0.5M NaCl to the reaction solution, add 20ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com