Method for preparing boron-doped titanium dioxide crystal containing specific crystal plane
A titanium dioxide and boron doping technology, applied in the field of photocatalytic materials, can solve the problems of not having high reaction selectivity and high visible light activity, and achieve high photocatalytic reaction selectivity, high visible light absorbance, and obvious advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
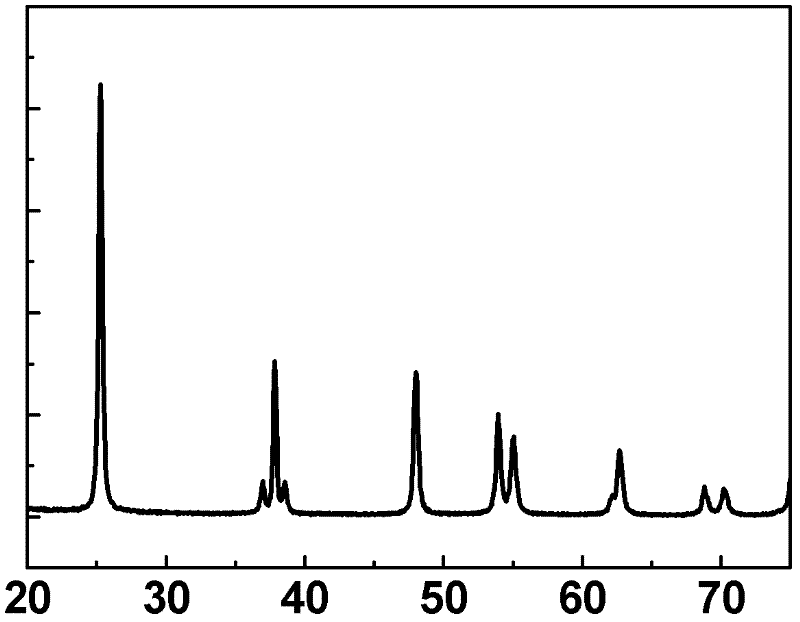
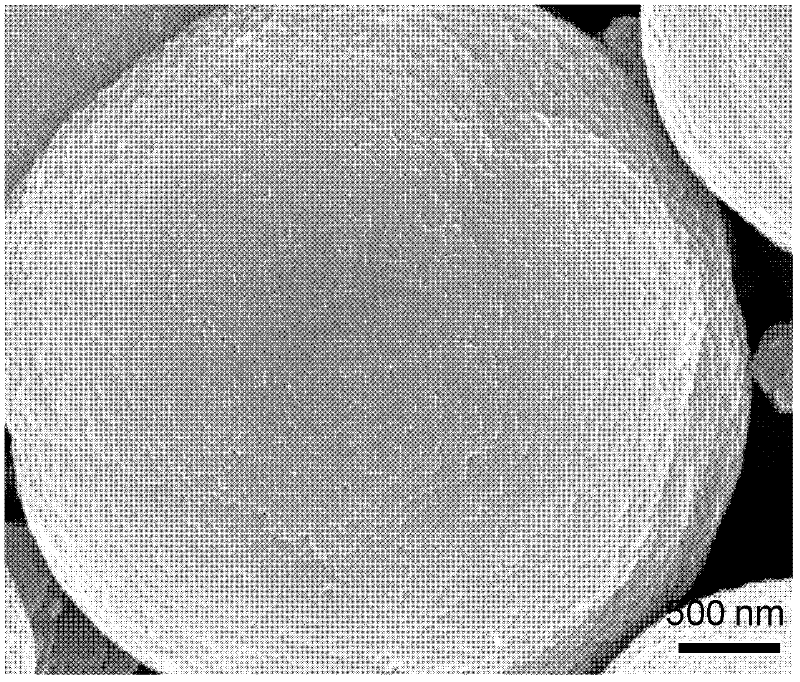
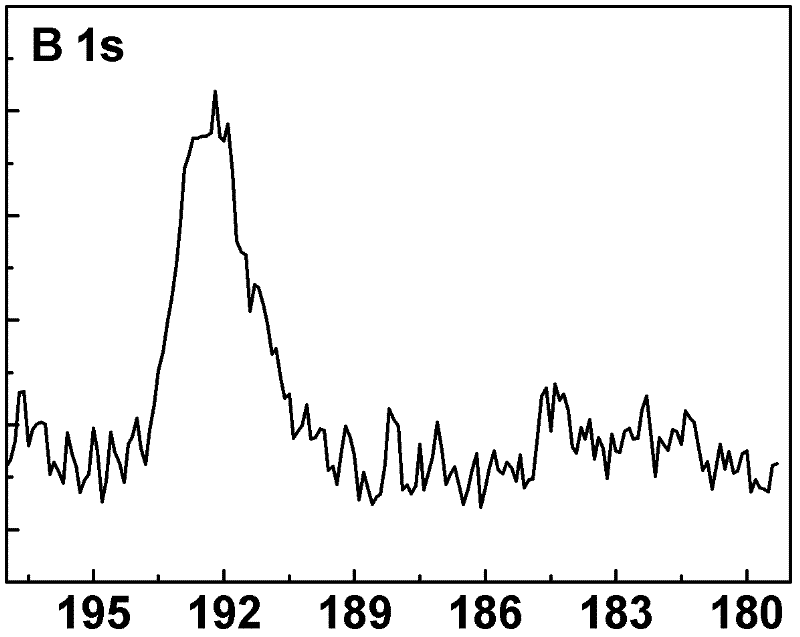
[0032] Weigh 80mg of commercial titanium boride powder (in this embodiment, the particle distribution range of titanium boride powder is 500nm-10μm, chemical formula TiB x , x=2), put it into 15mL containing 1.3M H + (In this example, H + Use H 2 SO 4 ), 0.8M SO 4 2- (In this embodiment, SO 4 2- Using Na 2 SO 4 ) in a 100mL stainless steel reactor lined with polytetrafluoroethylene. After the reaction kettle was sealed, it was put into an oven for heat treatment at 180°C for 24 hours, the reaction sample was taken out, washed with deionized water and dried at 80°C to obtain anatase titanium dioxide microspheres whose surface was mainly composed of {001} crystal facets. Treated in an air atmosphere at 600°C for 2 hours, the boron-doped anatase titanium dioxide rich in {001} crystal planes was obtained, and the {001} crystal planes accounted for more than 90%. In this embodiment, in the boron-doped titanium dioxide crystal, the doping amount of boron is 0.3 at%.
[00...
Embodiment 2
[0037]Weigh 200 mg of commercial titanium boride powder without any treatment (in this embodiment, the average particle size of titanium boride powder is 1000nm, chemical formula TiB x , x=1), put it into 20mL containing 2M H + (In this example, H + Using HCl), 2MCl - (In this embodiment, Cl - Adopt the water of KCl), ethanol mixed solution (water, ethanol volume ratio is 8: 1), in the 80mL stainless steel reaction kettle with polytetrafluoroethylene as lining. After the reactor was sealed, put it into an oven for heat treatment at 150°C for 24 hours, take out the reaction sample, wash it with deionized water and dry it at 80°C, and then heat-treat it in air at 700°C for 2 hours to obtain boron-doped and {111}-rich In the rutile titanium dioxide crystal with crystal plane, the {111} crystal plane accounts for more than 95%. In the boron-doped titanium dioxide crystal in this embodiment, the doping amount of boron is 0.4 at%.
[0038] Such as Figure 4 As shown, the prepa...
Embodiment 3
[0041] Weigh 350mg of commercial titanium boride powder without any treatment (in this embodiment, the particle distribution range of titanium boride powder is 500nm-10μm, chemical formula TiB x , x=2), put it into a container containing 50mL of 3M H + (In this example, H + Using HCl), 0.33M SO 4 - , 0.9MCl - (In this embodiment, Cl - Using NaCl, SO 4 2- Using Na 2 SO 4 ) in a 200mL stainless steel reactor lined with polytetrafluoroethylene. After the reaction kettle was sealed, it was placed in an oven for heat treatment at 200°C for 5 hours, and the reaction sample was taken out, washed with deionized water and dried at 80°C, and heat-treated at 500°C for 6 hours under a nitrogen atmosphere to obtain boron-doped anatase titanium dioxide crystals. Its surface is mainly composed of a high proportion of {101} crystal planes and a small proportion of {001} crystal planes, with {101} crystal planes accounting for 80% and {001} crystal planes accounting for 20%. In the b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com