Preparation method for epigallocatechin gallate (EGCG) methylated derivatives
A technology of methylation and derivatives, applied in the field of preparation of EGCG methylated derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
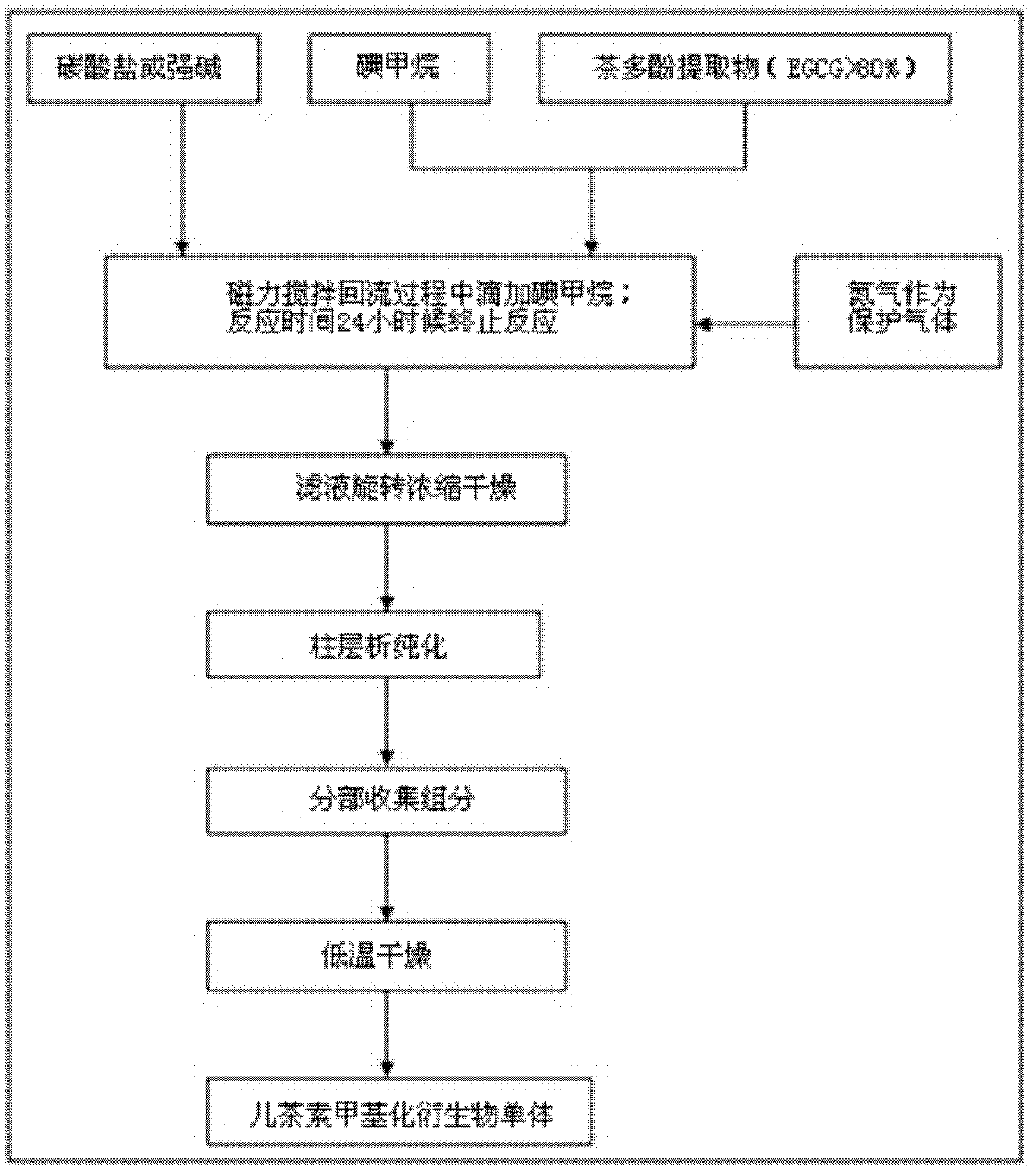
[0019] See figure 1 , The preparation method of the present invention is as follows:
[0020] (1) Add 10 grams of tea polyphenol extract with 98% EGCG content to 100 ml of acetone solution, then add 5 grams of sodium carbonate as a catalyst and nitrogen as a protective gas to form a reaction system;
[0021] (2) The reaction system was magnetically stirred and refluxed at 70°C, and methyl iodide was slowly added dropwise during the reflux process until the ratio of the amount of EGCG to methyl iodide reached 1:2; the reaction was terminated after 3 hours of reaction; The filtrate is concentrated and dried by rotating at low temperature to obtain a solid product;
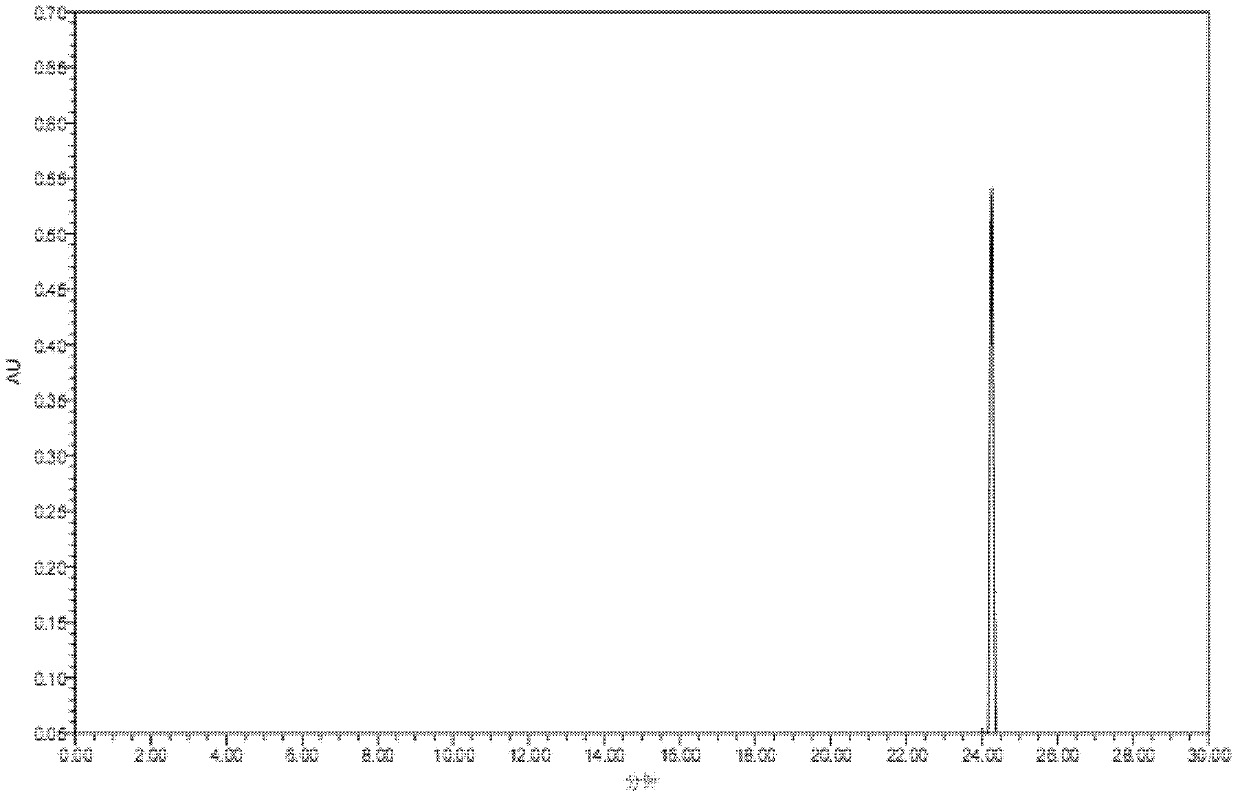
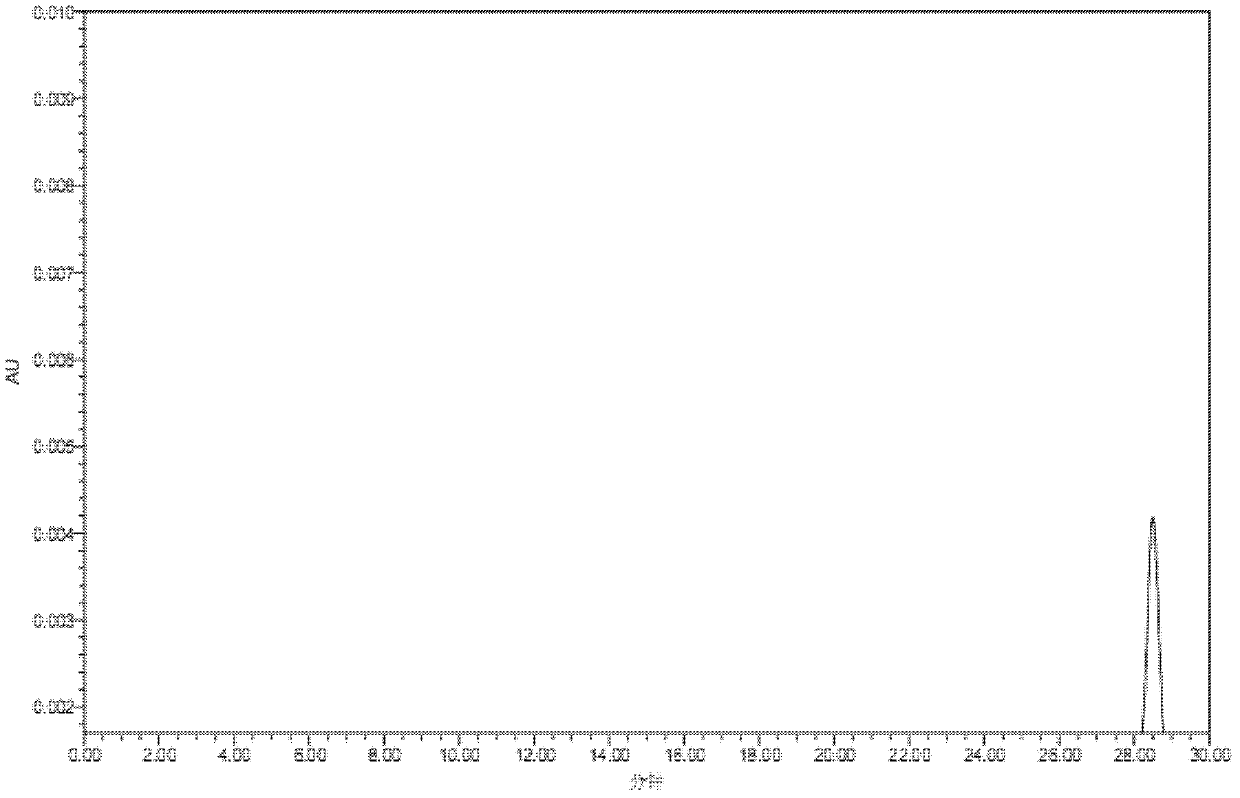
[0022] (3) After dissolving the above solid product in water, it passes through a chromatographic column containing Sephadex LH-20 material, and the flow rate of the chromatographic treatment is 3 bed volumes / hour; a methanol solution with a volume percentage of 10%-80% is used as The elution solvent is subjected to gradie...
Embodiment 2
[0026] See figure 1 , The preparation method of the present invention is as follows:
[0027] (1) Add 8 grams of tea polyphenol extract with 80% EGCG content to 100 ml of acetone solution, and then add 4 grams of sodium hydroxide as a catalyst and nitrogen as a protective gas to form a reaction system;
[0028] (2) The reaction system was magnetically stirred and refluxed at 75°C, while slowly adding methyl iodide during the refluxing process until the ratio of the amount of EGCG to methyl iodide reached 1:4; the reaction was terminated after 4 hours of reaction; The filtrate is concentrated and dried by rotating at low temperature to obtain a solid product;
[0029] (3) After dissolving the above solid product in water, it passes through a chromatography column containing Sephadex LH-20 material. The flow rate of the chromatography is 4 bed volumes / hour; a methanol solution with a volume percentage of 10%-80% is used as The elution solvent is used for gradient elution, and the flow...
Embodiment 3
[0033] See figure 1 , The preparation method of the present invention is as follows:
[0034] (1) Add 15 grams of tea polyphenol extract with 90% EGCG content to 200ml acetone solution, then add 7 grams of sodium carbonate as a catalyst and nitrogen as a protective gas to form a reaction system;
[0035] (2) The reaction system was magnetically stirred and refluxed at 70°C, while slowly adding methyl iodide during the refluxing process until the ratio of the amount of EGCG to methyl iodide reached 1:6; the reaction was terminated after 2 hours of reaction; The filtrate is concentrated and dried by rotating at low temperature to obtain a solid product;
[0036] (3) After dissolving the above solid product in water, it passes through a chromatographic column containing Sephadex LH-20 material, and the flow rate of the chromatographic treatment is 3 bed volumes / hour; a methanol solution with a volume percentage of 10%-80% is used as The elution solvent is subjected to gradient elution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com