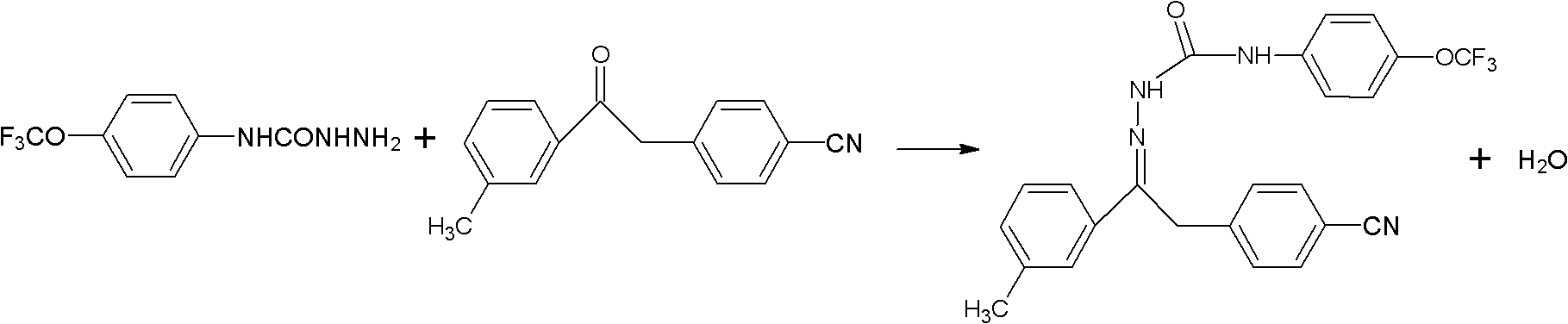
Method for synthesizing metaflumizone
A synthesis method and technology of cyflufenazone are applied in the field of synthesizing cyflufenazone, which can solve the problems of increased production cost, complicated post-processing, long production cycle, etc., and achieves improved yield, shortened reaction time, simplified post-processing and the like. effect of steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 23.53g m-trifluoromethylphenyl-4-cyanobenzyl ketone, 28.22g p-trifluoromethoxyanilinohydrazide, 1.17g concentrated hydrochloric acid, 282g dichloroethane, and react at 80°C to the reactor 1.5h, while reacting, use a water separator to separate the water generated by the reaction. After the reaction is completed, cool down to 0°C, filter, and dry to obtain 41.78g of product, with a total content of 96.0%, an effective body content of 95.7%, and a yield of was 92.3%.
Embodiment 2
[0021] Add 23.53g m-trifluoromethylphenyl-4-cyanobenzyl ketone, 23.52g p-trifluoromethoxyanilinohydrazide, 0.47g glacial acetic acid, 235g xylene, and react at 140°C for 2.5h into the reactor , while reacting, use a water separator to separate the water generated by the reaction. After the reaction is completed, cool down to 15°C, filter, and dry to obtain 40.89g of product, with a total content of 96.8%, an effective body content of 95.1%, and a yield of 90.4 %.
Embodiment 3
[0023] Add 23.53g of m-trifluoromethylphenyl-4-cyanobenzyl ketone, 24.70g of p-trifluoromethoxyanilinohydrazide, 0.59g of dilute sulfuric acid solution with a mass fraction of 2%, and 188g of toluene in the reactor , reacted at 110°C for 2 hours, and separated the water generated by the reaction with a water separator while reacting. After the reaction was completed, the temperature was lowered to 30°C, filtered, and dried to obtain 42.07g of the product, with a total content of 97.0% and an active body content of 96.0%. , the yield was 93.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com