Determination method for cobalt content in high-copper high-iron cobalt ores
A method of determination, cobalt ore technology, applied in the field of determination of cobalt content in cobalt ore, can solve problems affecting the accuracy of test results, etc., and achieve the effects of easy promotion, high precision, and large measurement range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
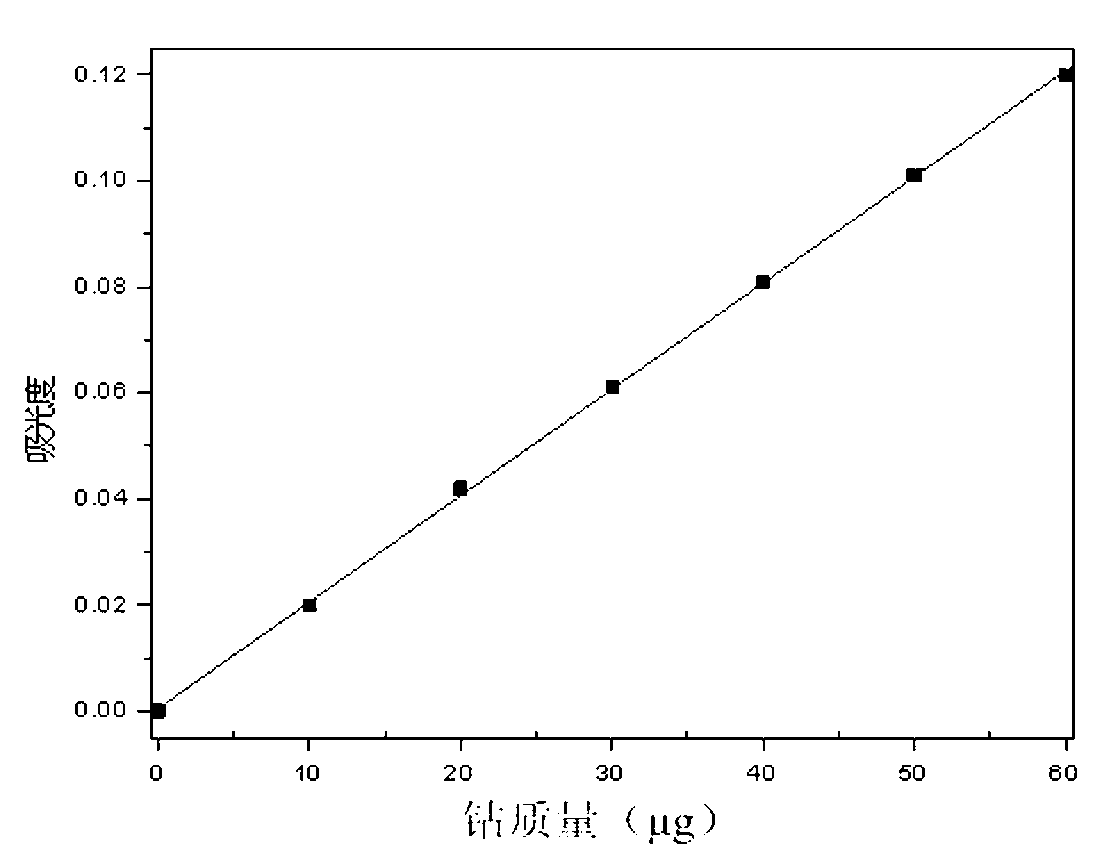
[0032] The weight content of iron in the selected cobalt ore sample is 5.364%, and the weight content of copper is 15.21%;
[0033] Take 0.1008g of cobalt ore sample in a 250mL beaker, add 20mL of aqua regia, and then heat it to 280°C to keep it warm. During the heat preservation process, yellow smoke is generated. When the yellow smoke disappears, stop heating to obtain the mineral sample solution;
[0034] Add 6mL mixed acid solution to the mineral sample solution, and then heat to 320°C. When the space above the mineral sample solution in the beaker is filled with white smoke, stop heating and cool to room temperature to obtain a shielded iron ore sample solution; the mixed acid solution is obtained by A sulfuric acid solution with a mass percentage of 98% and a phosphoric acid solution with a mass percentage of 85% are mixed at a volume ratio of 1:1;
[0035] Add 30mL of water to the shielded iron ore sample solution, heat it to 280°C, and then add a sodium thiosulfate sol...
Embodiment 2
[0044] The weight content of iron in the selected cobalt ore sample is 30.549%, and the weight content of copper is 22.376%;
[0045] Take 0.0820g of cobalt-containing ore sample in a 250mL beaker, add 55mL of aqua regia, and then heat it to 210°C to keep it warm. During the heat preservation process, yellow smoke is generated. When the yellow smoke disappears, stop heating to obtain the mineral sample solution;
[0046] Add 10mL mixed acid solution to the ore sample solution, and then heat to 350°C. When the space above the ore sample solution in the beaker is filled with white smoke, stop heating and cool to room temperature to obtain a shielded iron ore sample solution; the mixed acid solution is prepared by A sulfuric acid solution with a mass percentage of 98% and a phosphoric acid solution with a mass percentage of 85% are mixed at a volume ratio of 1:1;
[0047] Add 25mL of water to the shielded iron ore sample solution, heat to 200°C, and then add sodium thiosulfate so...
Embodiment 3
[0056] The weight content of iron in the selected cobalt ore sample is 34.93%, and the weight content of copper is 20.153%;
[0057] Take 0.2501g of cobalt ore sample in a 250mL beaker, add 60mL of aqua regia, and then heat it to 200°C to keep it warm. During the heat preservation process, yellow smoke is generated. When the yellow smoke disappears, stop heating to obtain the mineral sample solution;
[0058] Add 15mL mixed acid solution to the ore sample solution, and then heat to 300°C. When the space above the ore sample solution in the beaker is filled with white smoke, stop heating and cool to room temperature to obtain a shielded iron ore sample solution; the mixed acid solution is prepared by A sulfuric acid solution with a mass percentage of 98% and a phosphoric acid solution with a mass percentage of 85% are mixed at a volume ratio of 1:1;
[0059] Add 20mL of water to the shielded iron ore sample solution, heat to 230°C, and then add sodium thiosulfate solution with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com