Method for synthesizing aromatic nitrile with arylboronic acid
An arylboronic acid and aromatic nitrile technology, which is applied in the field of aromatic nitrile compound synthesis, can solve the problems of high cost and high toxicity of cyanating reagents, and achieve the effects of improving greenness, low toxicity and reducing production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
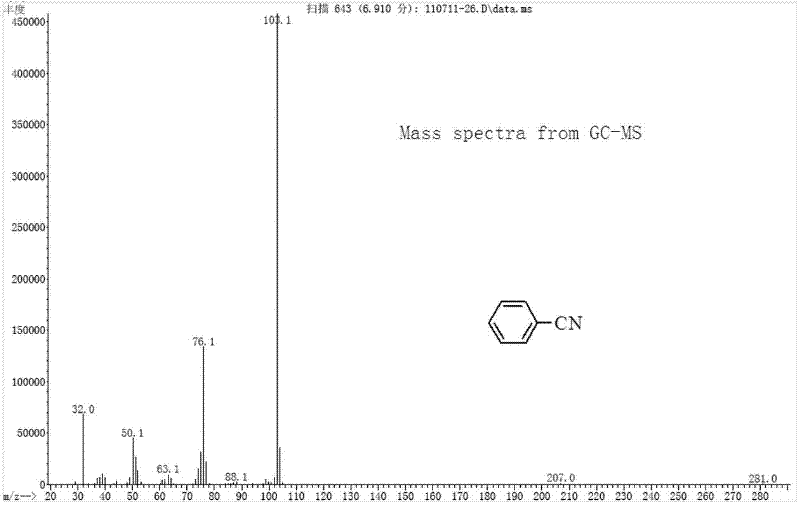
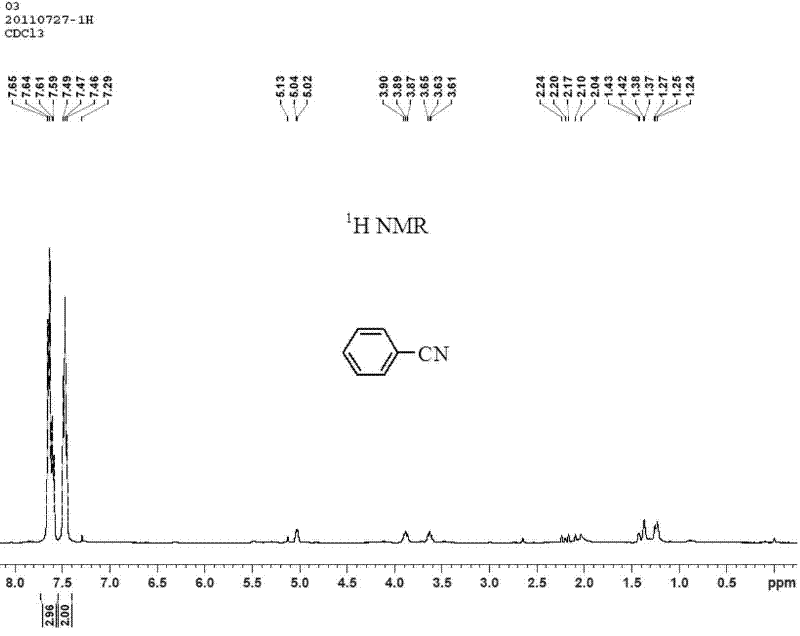
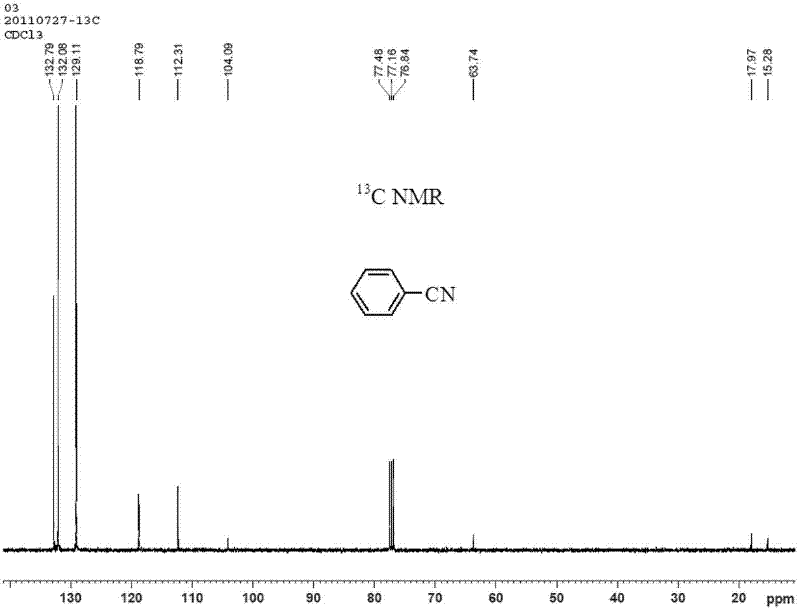
[0029] Under nitrogen protection, add catalyst (0.3mmol Cu(OAc) 2 ·H 2 O, 0.01 mmol Pd(OAc) 2 ), 1mmol K 2 CO 3 , 1mmol phenylboronic acid, 0.5 mmol K 4 [Fe(CN) 6 ], 1 mmol I 2 and 1.5 mL dimethyl sulfoxide, sealed the reaction vessel and placed it in an oil bath preheated to 160°C, and reacted for 6 hours under magnetic stirring. After the reaction, cool the reaction system to room temperature, add 1 mL of dichloromethane solution of phenylacetonitrile (concentration: 0.7 mmol / mL) as an internal standard, stir until mixed evenly, and let stand. The clear solution in the upper layer was absorbed, and the product was quantified by gas chromatography, and the yield of benzonitrile was 89%. The product was purified by column chromatography, using mass spectrometry, 1 H-NMR and 13 C-NMR confirmed the structure of the product. Benzonitrile (C 7 h 5 N) mass spectrometry (see figure 1 ): The theoretical value of the molecular ion peak (M+) is 103.04, and the measured va...
Embodiment 2
[0031] Under the protection of nitrogen, add 0.3mmol Cu(OAc) to the reaction vessel successively 2 ·H 2 O, 0.01 mmol Pd(OAc) 2 , 1 mmol K 2 CO 3 , 1mmol p-tolueneboronic acid, 0.5 mmol K 4 [Fe(CN) 6 ], 1 mmol I 2and 1.5 mL dimethyl sulfoxide, sealed the reaction vessel and placed it in an oil bath preheated to 160°C, and reacted for 6 hours under magnetic stirring. After the reaction, cool the reaction system to room temperature, add 1 mL of dichloromethane solution of phenylacetonitrile (concentration: 0.7 mmol / mL) as an internal standard, stir until mixed evenly, and let stand. The clear solution in the upper layer was sucked, and the product was quantified by gas chromatography, and the yield of p-methylbenzonitrile was 74%. The product was purified by column chromatography, using mass spectrometry, 1 H-NMR and 13 C-NMR confirmed the structure of the product. p-Toluonitrile (C 8 h 7 N) mass spectrometry (see Figure 4 ): The theoretical value of the molecular i...
Embodiment 3
[0033] Under the protection of nitrogen, add 0.3mmol Cu(OAc) to the reaction vessel successively 2 ·H 2 O, 0.01 mmol Pd(OAc) 2 , 1 mmol K 2 CO 3 , 1mmol m-methylphenylboronic acid, 0.5 mmol K 4 [Fe(CN) 6 ], 1 mmol I 2 and 1.5 mL dimethyl sulfoxide, sealed the reaction vessel and placed it in an oil bath preheated to 160°C, and reacted for 6 hours under magnetic stirring. After the reaction, cool the reaction system to room temperature, add 1 mL of dichloromethane solution of phenylacetonitrile (concentration: 0.7 mmol / mL) as an internal standard, stir until mixed evenly, and let stand. The clear solution in the upper layer was absorbed, and the product was quantified by gas chromatography, and the yield of m-methylbenzonitrile was 76%. The product was purified by column chromatography and its structure was confirmed by mass spectrometry. m-methylbenzonitrile (C 8 h 7 N) mass spectrometry (see Figure 5 ): The theoretical value of the molecular ion peak (M+) is 117.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com