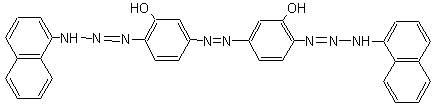
Method for preparing 3,3'-dihydroxy-4,4'-di(1,1'-dinaphthylamine azo group) azobenzene and application thereof
A technology of dinaphthylaminoazo and diaminoazobenzene, which is applied in the field of bistriazene compounds, can solve the problems of unsatisfactory reagent sensitivity and achieve the effects of high sensitivity, simple preparation method and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
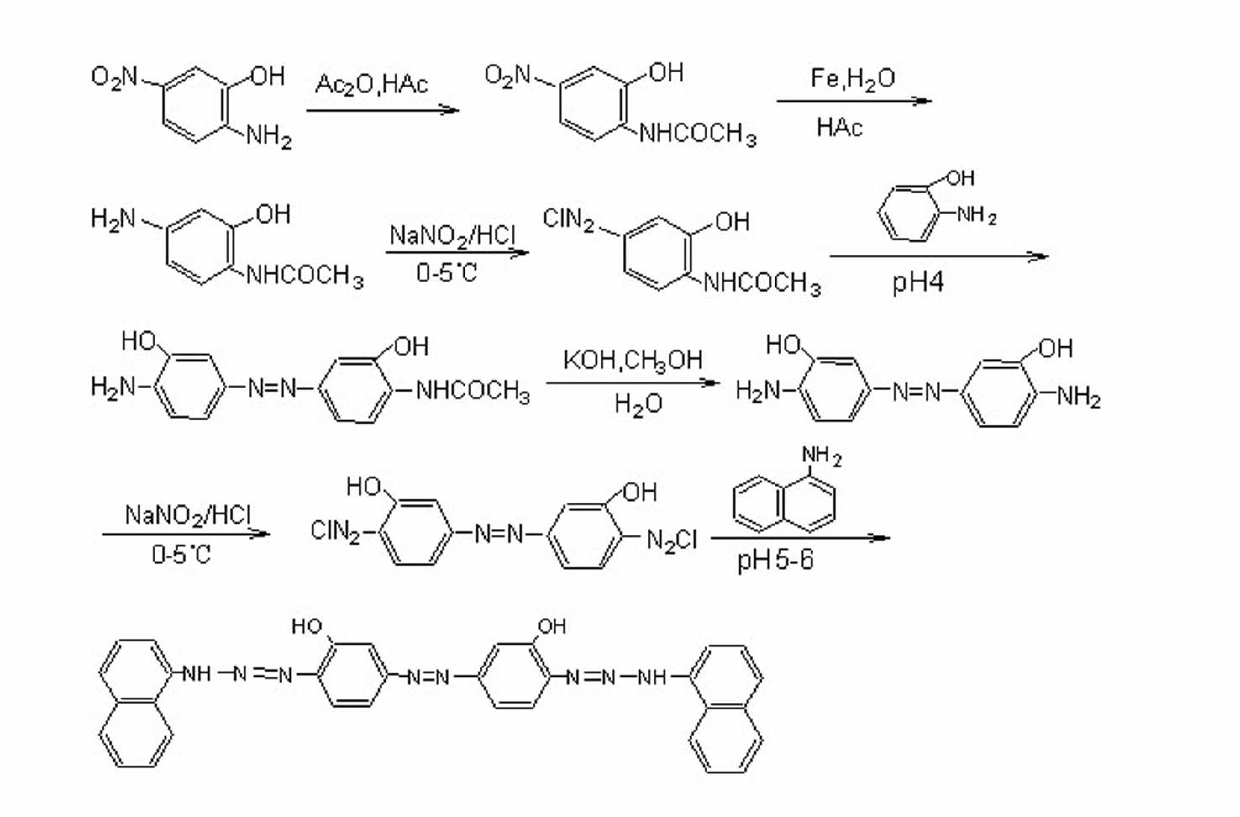
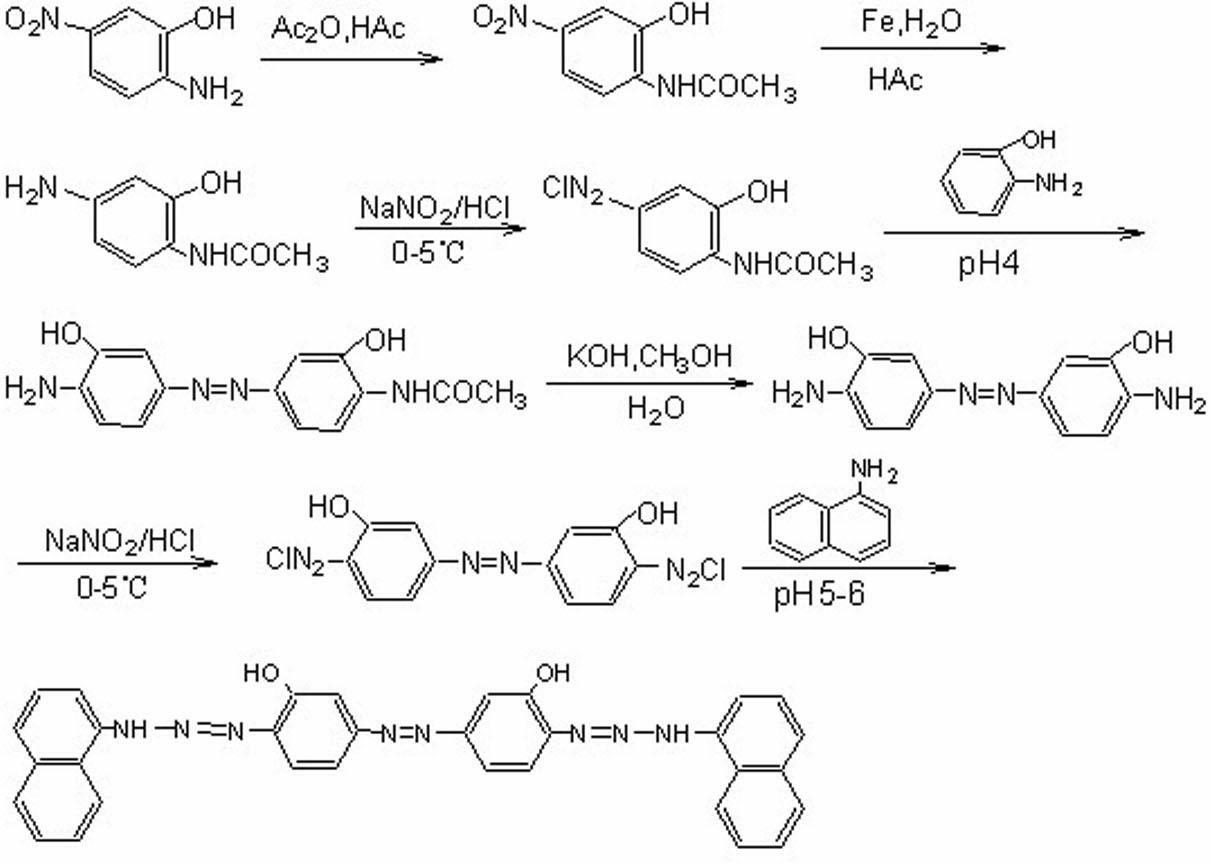
[0036] Example 1, a method for preparing 3,3′-dihydroxy-4,4′-bis(1,1′-dinaphthylaminoazo)azobenzene, comprising the following steps: as attached figure 1 to show
[0037] ① Acetylation of 2-hydroxy-4-nitroaniline In a 250ml round bottom flask equipped with a reflux condenser and a dropping funnel, add 60ml of glacial acetic acid and 30ml of acetic anhydride (0.31 mol), and add 2-hydroxyl -4-Nitroaniline 77 g (0.5 mol). Then slowly heat to reflux, then slowly add 27.2ml (0.29 mol) of acetic anhydride to the above solution dropwise, continue to reflux for 3h after the dropwise addition, and naturally cool to room temperature after the reaction is completed, add 150ml of ice water, and dissolve in sodium hydroxide neutralize, filter with suction, wash with water, and dry under an infrared lamp to obtain 94g of the product with a yield of 95%.
[0038] ② Reduction of 2-hydroxy-4-nitroacetanilide Add 400ml of distilled water into a 1000ml round bottom flask, heat to 65℃, then ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com