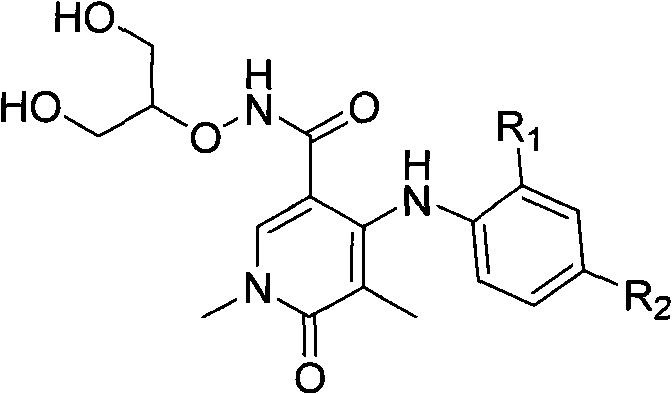
Micromolecular methyl ethyl ketone (MEK) protein kinase inhibitor
A compound and pharmaceutical technology, applied in the field of small-molecule MEK protein kinase inhibitors, can solve problems such as inability to bind
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
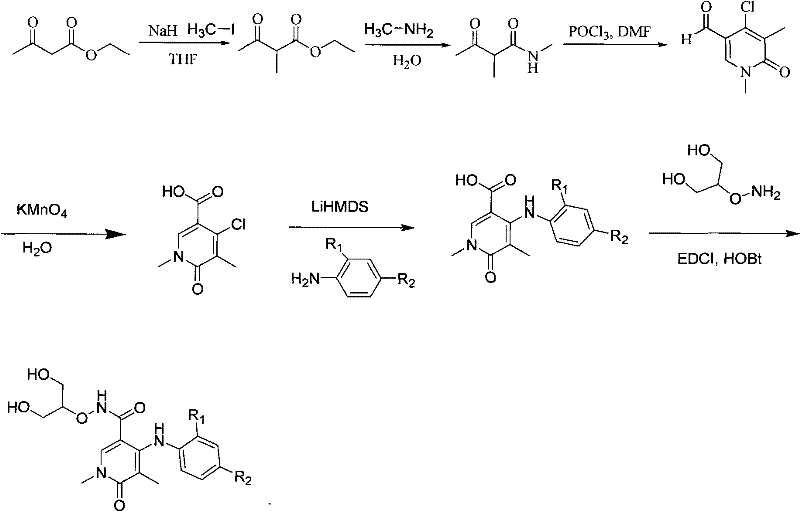
[0018] Example 1: 4-(2-fluoro-4-iodo-aniline)-1,5-bismethyl-6-oxo-1,6 dihydro-2H-pyridine-3-carboxylic acid (2-hydroxy- Synthesis of 1-hydroxymethyl-ethoxy)amide
[0019] The synthesis method is as follows:
[0020]
[0021] Step 1: Preparation of ethyl 2-methylacetoacetate
[0022] Dissolve ethyl acetoacetate (0.24mol) in THF (240mL), add NaH (0.264mol), stir at room temperature for 30min, N 2 For protection, add MeI (0.264mol) dropwise, after the addition was completed, overnight at 40°C, TLC showed that the raw material disappeared, adding water to quench the reaction, removing the solvent under reduced pressure, extracting with ethyl acetate, drying over anhydrous sodium sulfate, concentrating, distilling under reduced pressure, and collecting The distillate at 90°C / 0.1Mpa yielded 28g of light yellow liquid, yield: 81%. Mass spectrum: 145[(M+H) + ]
[0023] Step 2: Preparation of 2,N-dimethyl-3-oxo-butanamide
[0024] Add ethyl 2-methylacetoacetate (0.188mol) into...
Embodiment 2
[0033] Example 2: 4-(2-chloro-4-iodo-aniline)-1,5-bismethyl-6-oxo-1,6-dihydro-2H-pyridine-3-carboxylic acid (2-hydroxy -Synthesis of 1-hydroxymethyl-ethoxy)amide
[0034] The synthesis method is as follows:
[0035]
[0036] Step 1: Preparation of 4-(2-chloro-4-iodo-aniline)-1,5-bismethyl-6-oxo-1,6-dihydro-2H-pyridine-3-carboxylic acid
[0037] 4-Chloro-1,5-dimethyl-6-oxo-1,6-dihydro-2H-pyridine-3-acid (2g, 0.0099mol) was dissolved in THF, cooled to minus 78 degrees, added 19.8 ml 0.5M THF solution of lithium bis(trimethylsilyl)amide, and stirred at minus 78 degrees for half an hour. 2-Chloro-4-iodo-aniline (2.5 g, 0.0099 mol) was added, then warmed to room temperature and stirred overnight. Water was added, separated in a separatory funnel, the organic phase was dried and evaporated to dryness. The residue was separated with a silica gel column to obtain 4-(2-chloro-4-iodo-aniline)-1,5-bismethyl-6-oxo-1,6-dihydro-2H-pyridine-3-carboxylic acid 0.8g, yield: 19.3%. Mass...
Embodiment 3
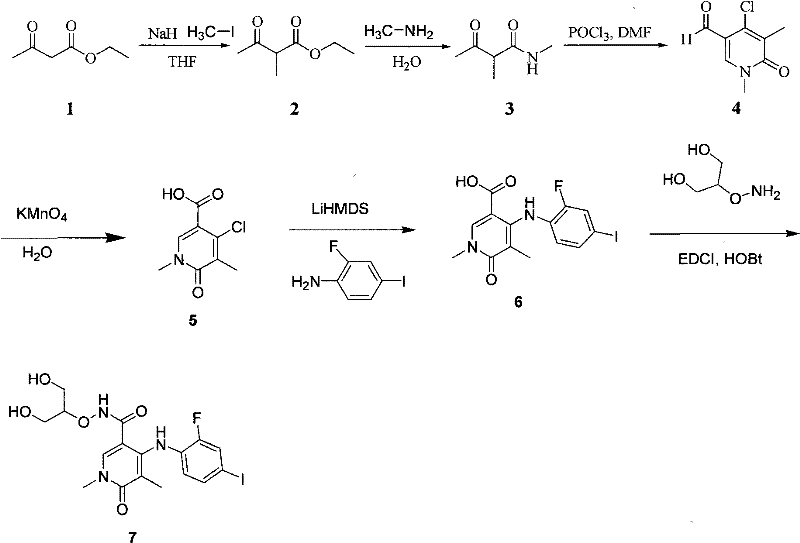
[0040] Example 3: 4-(2-fluoro-4-iodo-aniline)-1,5-bismethyl-6-oxo-1,6-dihydro-2H-pyridine-3-carboxylic acid (2-hydroxy Determination of Inhibition of MEK Protein Kinase Activity by -1-Hydroxymethyl-Ethoxy) Amide
[0041] With the assay kit of the MEK protein kinase inhibitor activity assay that buys from Sigma-Aldrich Company, analyze and get 4-(2-fluoro-4-iodo-aniline)-1,5-bismethyl-6-oxo- The half inhibitory dose of 1,6-dihydro-2H-pyridine-3-carboxylic acid (2-hydroxy-1-hydroxymethyl-ethoxy)amide to inhibit MEK protein kinase activity was 7nM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com