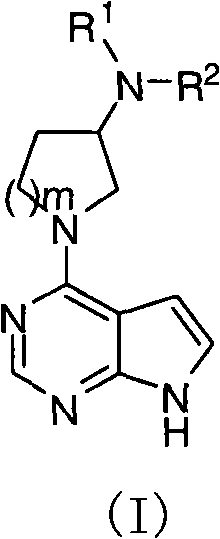
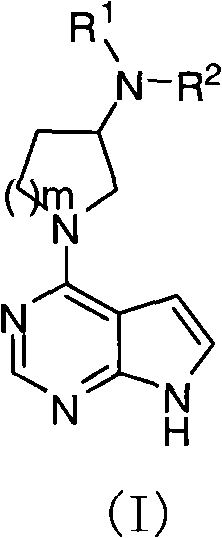
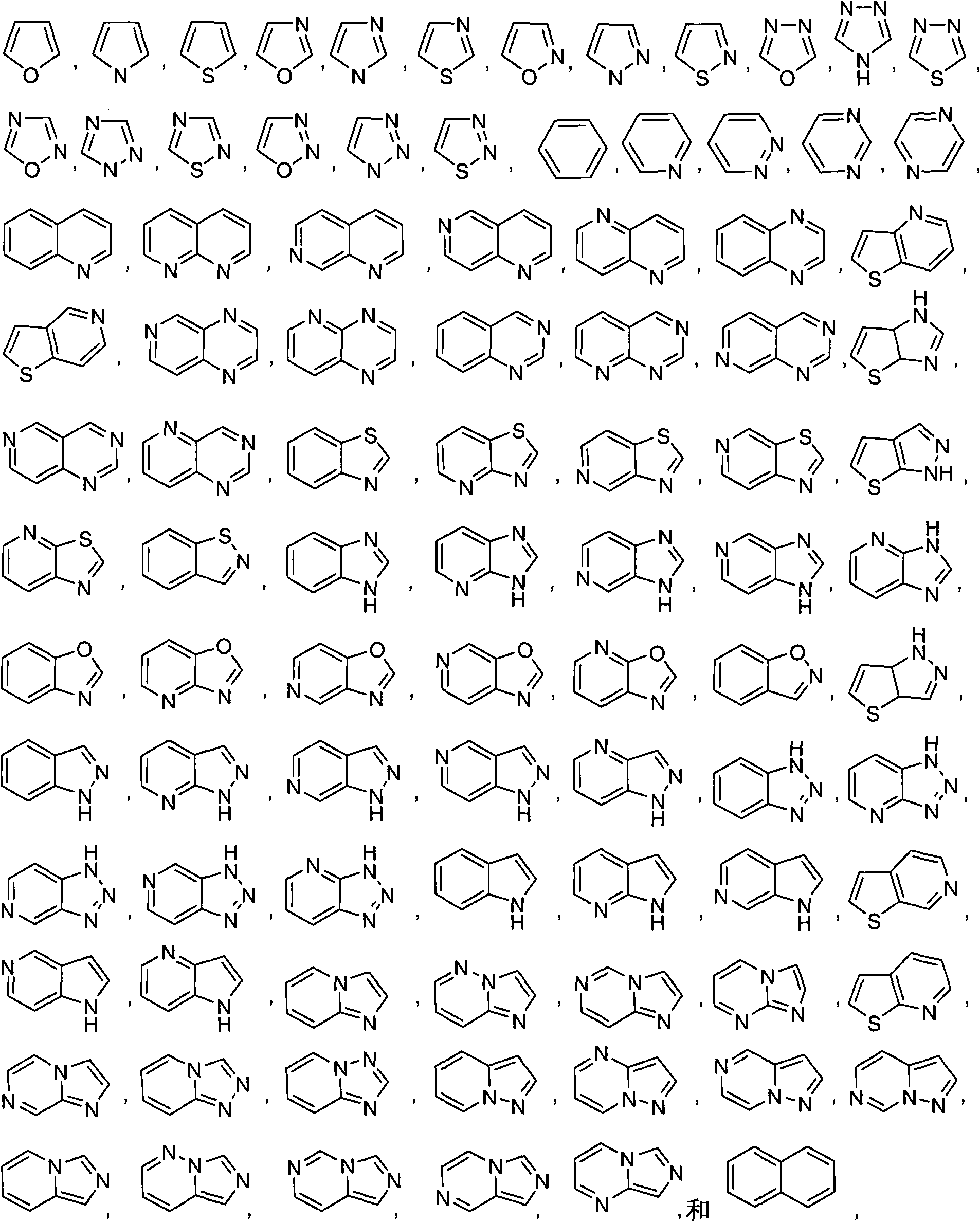
Pyrrolopyrimidine compound and use thereof
A compound and pharmaceutical technology, applied in the field of pyrrolopyrimidine compounds, can solve the problems of unclear gene mechanism and variation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0260] Embodiment 1: the synthesis of compound 1-260
[0261] Compound 1
[0262] (R)-N-(1-(7H-pyrrolo[2,3-d]pyrimidine-4-)pyrrolidine-3-)-2-cyano-N-methylacetamide
[0263]
[0264] (R)-N-Methyl-1-(7H-pyrrolo[2,3-d]pyrimidine-4-)pyrrolidin-3-amine (75mg, 0.345mmol) and cyanoacetic acid (35mg, 0.414mmol ) was dissolved in tetrahydrofuran (5 mL), HATU (157 mg, 0.414 mmol) and DIPEA (0.12 mL, 0.69 mmol) were added, and stirred at room temperature for 20 hours. The precipitated solid was filtered, the filter cake was washed with ethyl acetate and dried under reduced pressure to obtain the target product (45 mg, 46%). MS (m / z): 285 (M+H) + .
[0265] Compounds 2-8 can be prepared by those skilled in the art using corresponding intermediates and reagents under appropriate conditions, and the specific synthesis method can refer to compound 1.
[0266]
[0267]
[0268] Compound 9
[0269] (R)-N-(1-(7H-pyrrolo[2,3-d]pyrimidine-4-)pyrrolidine-3-)-4-cyano-N-methylbenzenesul...
Embodiment 2
[0525] Enzyme activity detection
[0526] With Z'-LYTE TM Kinase Assay Kit-Tyr 6 Peptide (Invitrogen, Cat. No. PV4122) kit was used to analyze JAK1 / 2 / 3 kinase activity. With Z'-LYTE TM Kinase Assay Kit-Tyr 3Peptide (Invitrogen, Cat. No. PV3192) kit was used to analyze TYK2 kinase activity. Recombinant human JAK1 / 2 / 3 or TYK2 kinases were purchased from Invitrogen (Cat No. PV4774 / PV4210 / PV3855 / PV4790); 20 μL total reaction solution as follows, containing 2.5 μL test compound dissolved in 4% DMSO, 5 μL enzyme / substrate mix Buffer (containing 3.2, 0.04, 0.2, 8 μg / mL recombinant human JAK1 / 2 / 3 or TYK2 kinase, 4 μM Tyr 6 or Tyr 3 reaction substrate peptide) or Tyr 6 or Tyr 3 phosphorylation substrate buffer (Invitrogen, Cat.No.PV3192, diluted with 1.33x kinase buffer), 2.5 μL ATP solution (300 / 100 / 40 / 100 μM, JAK1 / 2 / 3 or TYK2 kinase) or 1.33x kinase buffer (Invitrogen, Cat.No.PV3189 , 5x kinase buffer, diluted with water). Mix the components in the reaction wells (total volume...
Embodiment 3
[0529] cell detection
[0530] In order to detect IL6-induced phosphorylated STAT3 levels, HepG2 cells (SIBS) were resuspended in serum-free DMEM medium at 5.4×10 3 Cells were seeded in 96-well plates and incubated overnight in a cell culture incubator. The next day, various concentrations of compounds were added, and after incubation for 30 minutes, human recombinant IL6 with a final concentration of 10 ng / ml was added to stimulate for 15 minutes. The cells were then fixed with 2% paraformaldehyde at room temperature for 45 minutes, incubated with ice-cold methanol for another 30 minutes, washed with PBS, and incubated with rabbit anti-phosphorylated STAT3 (Y705) (Cell Signaling Technologies) primary antibody overnight at 4°C. After washing, incubate with goat anti-rabbit IgG Alexa 488 secondary antibody for 90 minutes. Add 7.5uM propidium iodide (containing 100g / ml RNaseA) and stain in the dark for 60 minutes, and read the plate with an Acumen X3 (TPPLabtech) instrument. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com