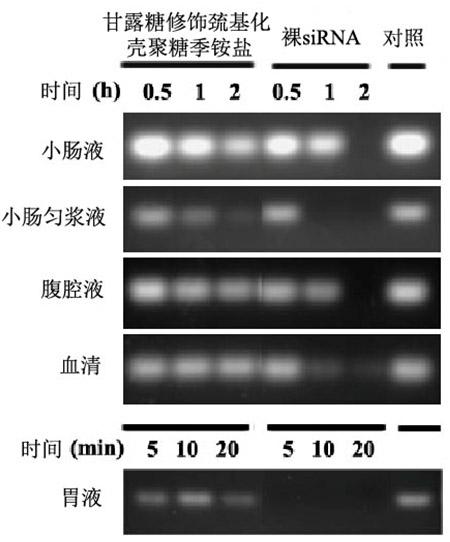
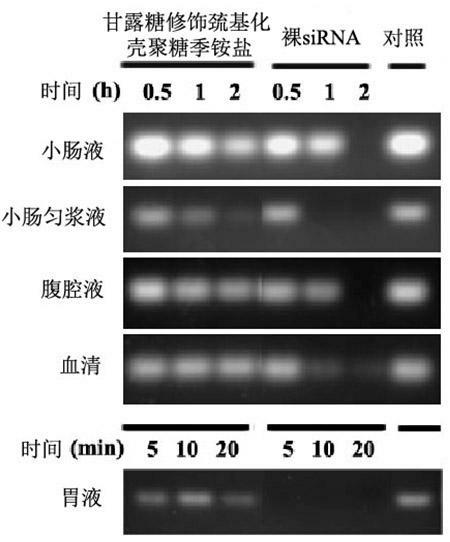
Mannose-modified thiolated chitosan quaternary ammonium salt nanoparticle, preparing method and application thereof
A technology of thiolated chitosan and chitosan quaternary ammonium salt, which is applied in the field of nanoparticles, can solve the problems of inability to effectively improve the uptake of small intestinal epithelial cells, lack of targeting specific groups, lower absorption and transfection efficiency, etc. problems, to achieve the effect of improving cell adhesion, increasing affinity, and increasing oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
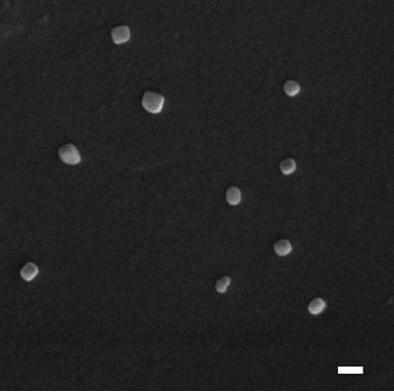
[0057] Example 1 Preparation of mannose-modified chitosan quaternary ammonium salt-cysteine
[0058] TMC was synthesized according to the method reported in the literature (Eur J Pharm Biopharm 2004,57:77-83). The weight average molecular weight of chitosan was 30 kDa, and the reaction time was 45 min, 120 min and 180 min, respectively. The degree of quaternization of TMC was respectively 15%, 30% and 60%. Cl - Purified by anion-exchange resin, freeze-dried; weighed 400 mg TMC dissolved in 160 mL water, 400 mg mannopyranyl isothiocyanate dissolved in 40 mL dimethyl sulfoxide (DMSO), mixed, adjusted to pH 8.5 , reacted at room temperature for 6 h, ultrafiltered (MWCO 10 kDa), and the obtained Man-TMC was freeze-dried; weighed 160 mg Man-TMC dissolved in 8 mL water, 5 mg cysteine was dissolved in 2 mL water, mixed, and added to the final The concentrations of EDAC and NHS were 50 mM, adjusted to pH 5.0, and reacted at room temperature for 5 h. The reaction product was dialyz...
Embodiment 2
[0059] Example 2 Preparation of mannose-modified chitosan quaternary ammonium salt-cysteine
[0060] TMC was synthesized according to the method reported in the literature (Eur J Pharm Biopharm 2004,57:77-83). The weight average molecular weight of chitosan was 100 kDa, and the reaction time was 45 min, 120 min and 180 min, respectively. 15%, 30% and 60%. Cl - Purified by anion-exchange resin, freeze-dried; weighed 400 mg TMC dissolved in 160 mL water, 400 mg mannopyranyl isothiocyanate dissolved in 40 mL dimethyl sulfoxide (DMSO), mixed, adjusted to pH 9.0 , reacted at room temperature for 12 h, ultrafiltered (MWCO 10 kDa), and the obtained Man-TMC was freeze-dried; weighed 125 mg Man-TMC dissolved in 8 mL water, 5 mg cysteine was dissolved in 2 mL water, mixed, and added to the final The concentrations of EDAC and NHS were 100 mM, adjusted to pH 3.0, and reacted at room temperature for 2 h. The reaction product was dialyzed against pH 5.0 HCl solution (MWCO 3500 Da) at 4...
Embodiment 3
[0061] Example 3 Preparation of mannose-modified chitosan quaternary ammonium salt-cysteine
[0062] TMC was synthesized according to the method reported in the literature (Eur J Pharm Biopharm 2004,57:77-83). The weight average molecular weight of chitosan was 200 kDa, and the reaction time was 45 min, 120 min and 180 min, respectively. The quaternization degrees of TMC were respectively 15%, 30% and 60%. Cl - Purified by anion-exchange resin, freeze-dried; Weigh 400 mg TMC dissolved in 160 mL water, 400 mg mannopyranyl isothiocyanate dissolved in 40 mL dimethyl sulfoxide (DMSO), mix, adjust pH to 9.5 , reacted at room temperature for 24 h, ultrafiltered (MWCO 10 kDa), and the obtained Man-TMC was freeze-dried; weighed 100 mg Man-TMC dissolved in 8 mL water, 5 mg cysteine dissolved in 2 mL water, mixed, and added to the final The concentrations of EDAC and NHS were 200 mM, adjusted to pH 4.0, and reacted at room temperature for 8 h. The reaction product was dialyzed again...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com