Pyrazole tetrazole carboxamide and synthesis method thereof
A technology of pyrazole tetrazolium amide and phenylpyrazole amide, which is applied in the field of pyrazole tetrazolium amide derivatives and their microwave synthesis, can solve the problems of high energy consumption, long reaction time, and many side reactions. Achieve the effect of short reaction time, high yield and high killing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
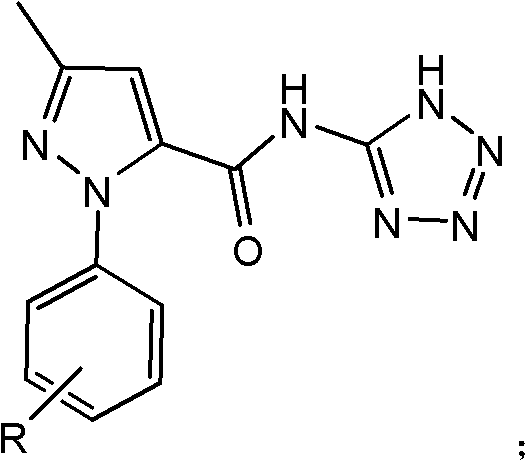
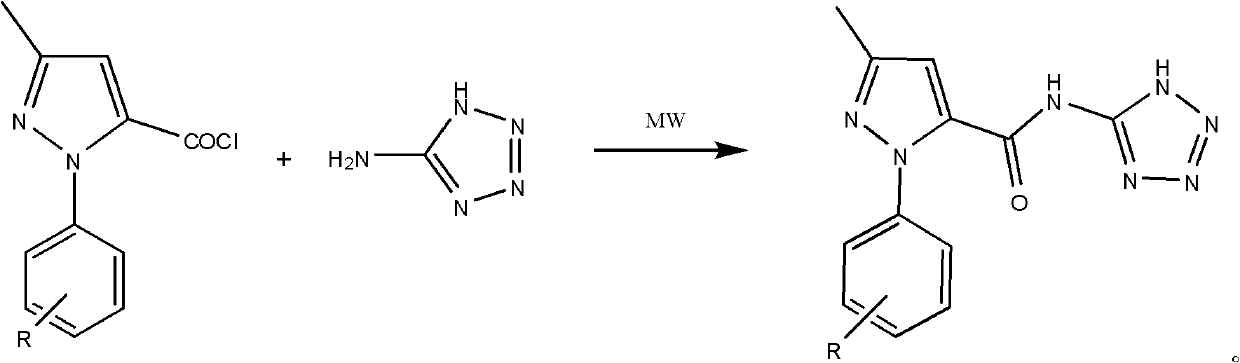
[0024] Synthesis of 3-methyl-1-phenyl-N-(1-hydrogen-tetrazol-5-yl)-1-hydrogen-pyrazole-5-amide
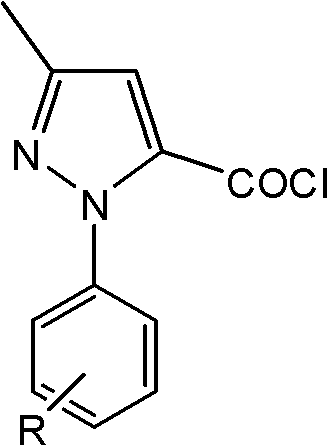
[0025] In a 250ml four-neck flask, add 0.01mol of 3-methyl-1-phenyl-1-hydrogen-pyrazole-5-acyl chloride, 0.01mol of 5-aminotetrazole, dissolve in 100ml of toluene, and irradiate the mixture under 400W microwave Under the light, heat to 110°C, react for 20min, cool and filter. The product was obtained by recrystallization with ethanol, the yield: 85%. Melting point: 223~225℃.
[0026]
[0027] 1 H NMR (DMSO-d6, 300MHz): 2.27(s, 3H, CH 3 ), 6.83(s, 1H, pyrazole C-H), 7.38-7.41(m, 5H, Ar-H), 9.39-9.41(t, 1H, -NH-CO-), 14.26(s, 1H, tetrazole N-H).
[0028] IR (cm -1 ): 3131, 2976, 1701, 1658, 1314.
[0029] The bactericidal activity test shows that: when the concentration is 10ppm, the bactericidal rate of sulfate-reducing bacteria and iron bacteria reaches 90.9% and 76% respectively in 1 hour.
Embodiment 2
[0031] Synthesis of 3-methyl-N-(1-hydro-tetrazol-5-yl)-1-p-tolyl-1-hydro-pyrazole-5-amide.
[0032] In a 250ml four-neck flask, add 0.01mol of 3-methyl-1-p-tolyl-1-hydro-pyrazole-5-acyl chloride), 0.012mol of 5-aminotetrazole, dissolve in 100ml of ethanol, and Under microwave irradiation, heat to 115°C, react for 5 minutes, cool and filter. The product was obtained by recrystallization with toluene, yield: 73%. Melting point: 246-247°C.
[0033]
[0034] 1 H NMR (DMSO-d6, 300MHz): 2.24(s, 3H, CH 3 ), 2.27 (s, 3H, CH 3 ), 6.83 (s, 1H, pyrazole C-H), 7.19-7.36 (m, 4H, Ar-H), 9.41 (d, 1H, -NH-CO-), 14.19 (s, 1H, tetrazole N-H) .
[0035] IR (cm -1 ): 3140, 2972, 1699, 1606, 1377.
[0036] The bactericidal activity test shows that: when the concentration is 10ppm, the bactericidal rate of sulfate-reducing bacteria and iron bacteria reaches 92.2% and 78.3% respectively in 1h.
Embodiment 3
[0038] Synthesis of 1-(4-methoxyphenyl)-3-methyl-N-(1-hydrogen-tetrazol-5-yl)-1-hydrogen-pyrazole-5-amide
[0039] In a 250ml four-neck flask, add 0.01mol of 1-(4-methoxyphenyl)-3-methyl-1-hydrogen-pyrazole-5-acid chloride, 0.015mol of 5-aminotetrazole, dissolve in 100ml of toluene , under 300W microwave irradiation, heated to 110°C, reacted for 15min, cooled and filtered. The product was obtained by recrystallization from ethanol, with a yield of 82%. Melting point: 229-231°C.
[0040]
[0041] 1 H NMR (DMSO-d6, 300MHz): 2.24(s, 3H, CH 3 ), 3.80 (s, 3H, OCH 3 ), 6.83 (s, 1H, pyrazole C-H), 7.10-7.35 (m, 4H, Ar-H), 9.41 (d, 1H, -NH-CO-), 14.26 (s, 1H, tetrazole N-H) .
[0042] IR (cm -1 ): 3134, 2964, 1691, 1602, 1312.
[0043] The bactericidal activity test shows that: when the concentration is 10ppm, the bactericidal rates of sulfate-reducing bacteria and iron bacteria reach 91.3% and 80% respectively in 1 hour.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com