Process for producing potassium N-methyldithiocarbamate
A technology of methyl dithiocarbamic acid and methyl dithioamino group, which is applied in the field of production technology of potassium N-methyldithiocarbamate, can solve the problems of low degree of agricultural mechanization, and achieve the goal of using Sustainability, high concentration, and operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
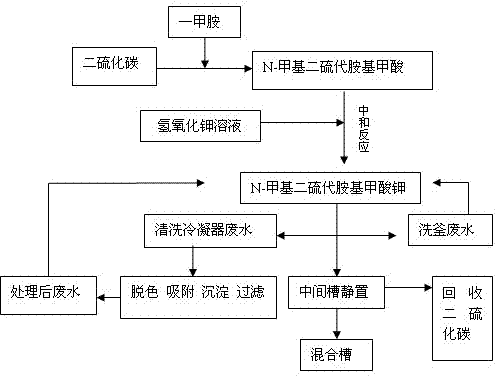
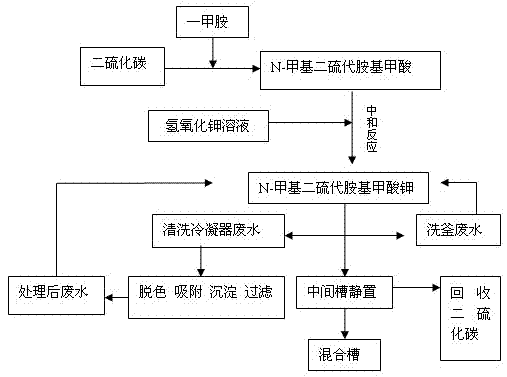
[0018] combined with figure 1 To specifically illustrate the production process of the present invention.
[0019] First, 40%wt monomethylamine aqueous solution containing 0.95 mole of monomethylamine was gradually added dropwise into 1.05 mole of carbon disulfide and reacted at 15~45°C for 2~3h to obtain N-methyldithiocarbamic acid. Since this reaction is an exothermic reaction, it is necessary to increase cooling and control the rate of addition during the reaction to ensure that the reaction temperature does not exceed 45°C.
[0020] Then, add 30%wt potassium hydroxide aqueous solution containing 1.0 moles of potassium hydroxide and carry out neutralization reaction at 35~45°C for 3h to obtain N-methyldithiocarbamic acid potassium solution to obtain N-methyldithiocarbamate The mass percent concentration of the potassium carbamate solution was 52.3%, and the yield was 98.0%. Then, after putting the reaction solution into the middle tank of the cone and standing for stratif...
Embodiment 2
[0024] combined with figure 1 To specifically illustrate the production process of the present invention.
[0025] First, 40%wt monomethylamine aqueous solution containing 0.97 mole of monomethylamine was gradually added dropwise into 1.1 mole of carbon disulfide and reacted at 15~45°C for 2~3h to obtain N-methyldithiocarbamic acid. Since this reaction is an exothermic reaction, it is necessary to increase cooling to control the rate of addition during the reaction, so as to ensure that the reaction temperature does not exceed 45°C.
[0026] Then, add 30% potassium hydroxide aqueous solution containing 1.02 moles of potassium hydroxide and carry out neutralization reaction at 35~45°C for 2 hours to obtain N-methyldithiocarbamic acid potassium solution to obtain N-methyldithioamine The mass percentage concentration of potassium formate solution is 55.6%, yield 98.3%
[0027] Then, after putting the reaction solution into the middle tank of the cone and standing for stratifica...
Embodiment 3
[0031] combined with figure 1 To specifically illustrate the production process of the present invention.
[0032] First, 40%wt monomethylamine aqueous solution containing 0.99 mole of monomethylamine was gradually added dropwise into 1.2 mole of carbon disulfide and reacted at 15~45°C for 2~3h to obtain N-methyldithiocarbamic acid. Since this reaction is an exothermic reaction, it is necessary to increase cooling to control the rate of addition during the reaction, so as to ensure that the reaction temperature does not exceed 45°C.
[0033] Then, add 30% potassium hydroxide aqueous solution containing 1.05 moles of potassium hydroxide and carry out neutralization reaction at 35~45°C for 2 hours to obtain N-methyldithiocarbamic acid potassium solution to obtain N-methyldithioamine The mass percentage concentration of potassium methionate solution is 60%, yield 98.9%
[0034] Then, after putting the reaction solution into the middle tank of the cone and standing for stratific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com