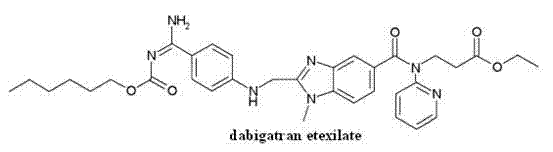
Dabigatran compound and preparation method and medicinal composition thereof
A technology of dabigatran etexilate and dabigatran etexilate mesylate, which is applied in the field of medicine, can solve the problems of many interfering factors, large individual differences in dosage, and narrow treatment window, and achieve easy operation, few types of use, Conducive to the effect of production and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of dabigatran etexilate mesylate:
[0032] With 6.278g (0.01mol) dabigatran etexilate (method as described in WO 98 / 37075), be dissolved in 400ml ethyl acetate, add the acetic acid that dissolves methanesulfonic acid 0.961g (0.01mol) while stirring at room temperature 40ml of ethyl ester solution, continue to stir for 60 minutes after the addition, then put it in an ice bath and stir for 40 minutes, filter, wash the filter cake with 80ml of ethyl acetate, dry in a circulating air drier at 55°C for 3h, and obtain 6.85g of Bigatran etexilate mesylate anhydrate, yield 94.6%. Melting point: 178-179°C.
[0033] Elemental analysis:
[0034] Elemental analysis Actual value% Theoretical value% C 58.02 58.09 H 6.32 6.22 N 13.60 13.55 O 17.24 17.27 S 4.47 4.43
Embodiment 2
[0035] . Embodiment 2: the preparation of dabigatran etexilate compound of the present invention:
[0036] Take 2 g of dabigatran etexilate methanesulfonate in Example 1, add 100 ml of hot water at 60°C to dissolve, cool to 15-20°C and stir for 1 hour, then cool to 5-10°C and stir for 1 hour, and finally cool to -5 Stirring at -0°C for 10 hours, crystals were precipitated, filtered, and the filter cake was dried at 35°C and 65% relative humidity for 6 hours to obtain 1.68 g of the dabigatran etexilate compound of the present invention, with a yield of 82%.
[0037] Elemental analysis:
[0038] Elemental analysis Actual value% Theoretical value% C 56.56 56.68 H 6.38 6.34 N 13.25 13.22 O 19.46 19.43 S 4.27 4.32
[0039] The moisture in the dabigatran etexilate compound of the present invention measured by Karl Fischer method is 2.2% (theory: 2.4%); the thermogravimetric analysis result shows that it is the characteristic of monohy...
Embodiment 3
[0040] Embodiment 3: the preparation of dabigatran etexilate compound of the present invention:
[0041] Take 2g of dabigatran etexilate methanesulfonate in Example 1, add 100ml of hot water at 60°C to dissolve, cool to 15-20°C and stir for 1 hour, then cool to 5-10°C and stir for 1 hour, and finally cool to - Stir at 5-0°C for 10 hours to precipitate crystals, filter, and dry the filter cake at 30°C and 60% relative humidity for 8 hours to obtain 1.63 g of the dabigatran etexilate compound of the present invention, with a yield of 80%.
[0042] Elemental analysis:
[0043] Elemental analysis Actual value% Theoretical value% C 56.61 56.68 H 6.29 6.34 N 13.28 13.22 O 19.51 19.43 S 4.26 4.32
[0044]The moisture in the dabigatran etexilate compound of the present invention measured by Karl Fischer method is 2.5% (theory: 2.4%); the thermogravimetric analysis result shows that it is the characteristic of monohydrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com