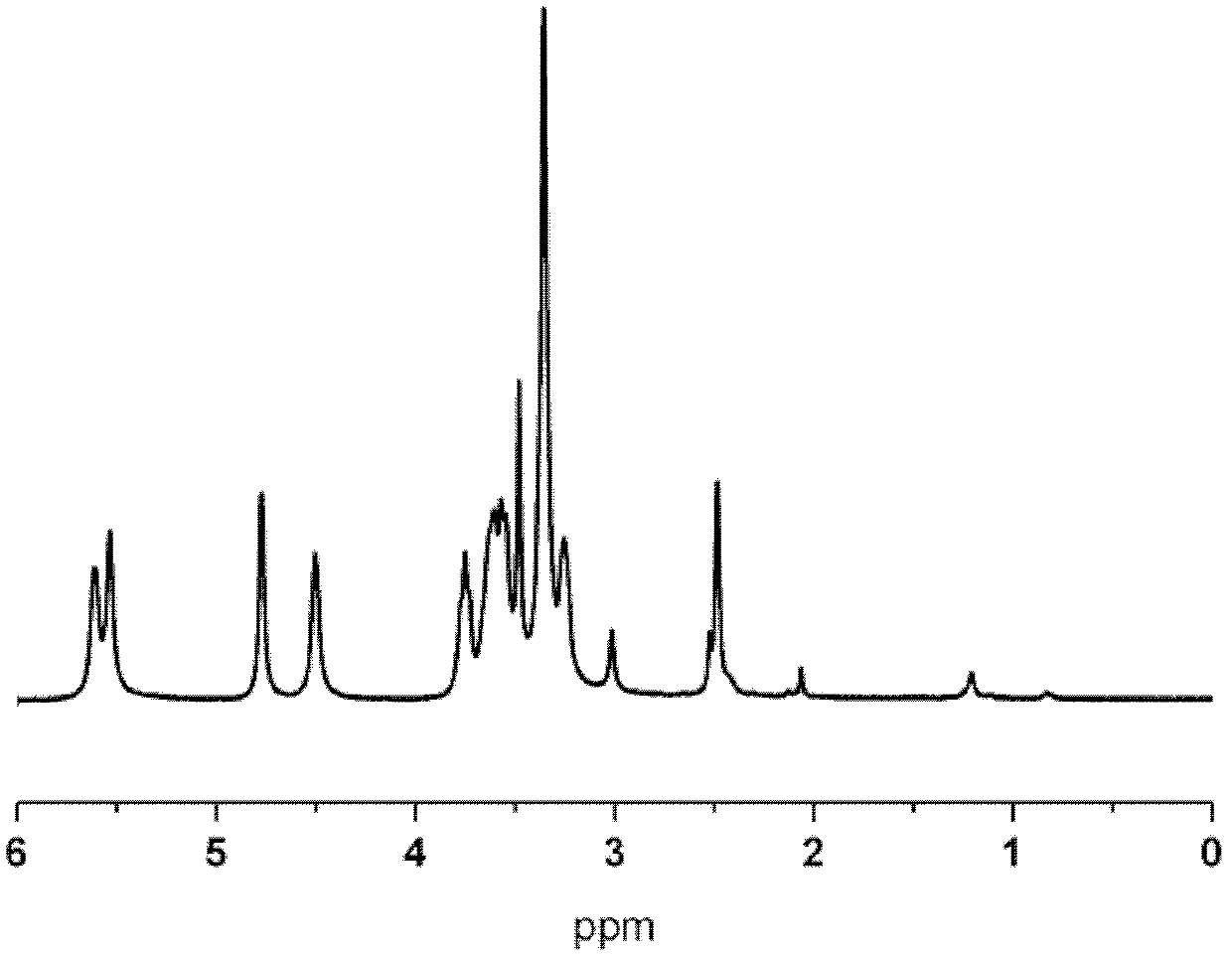
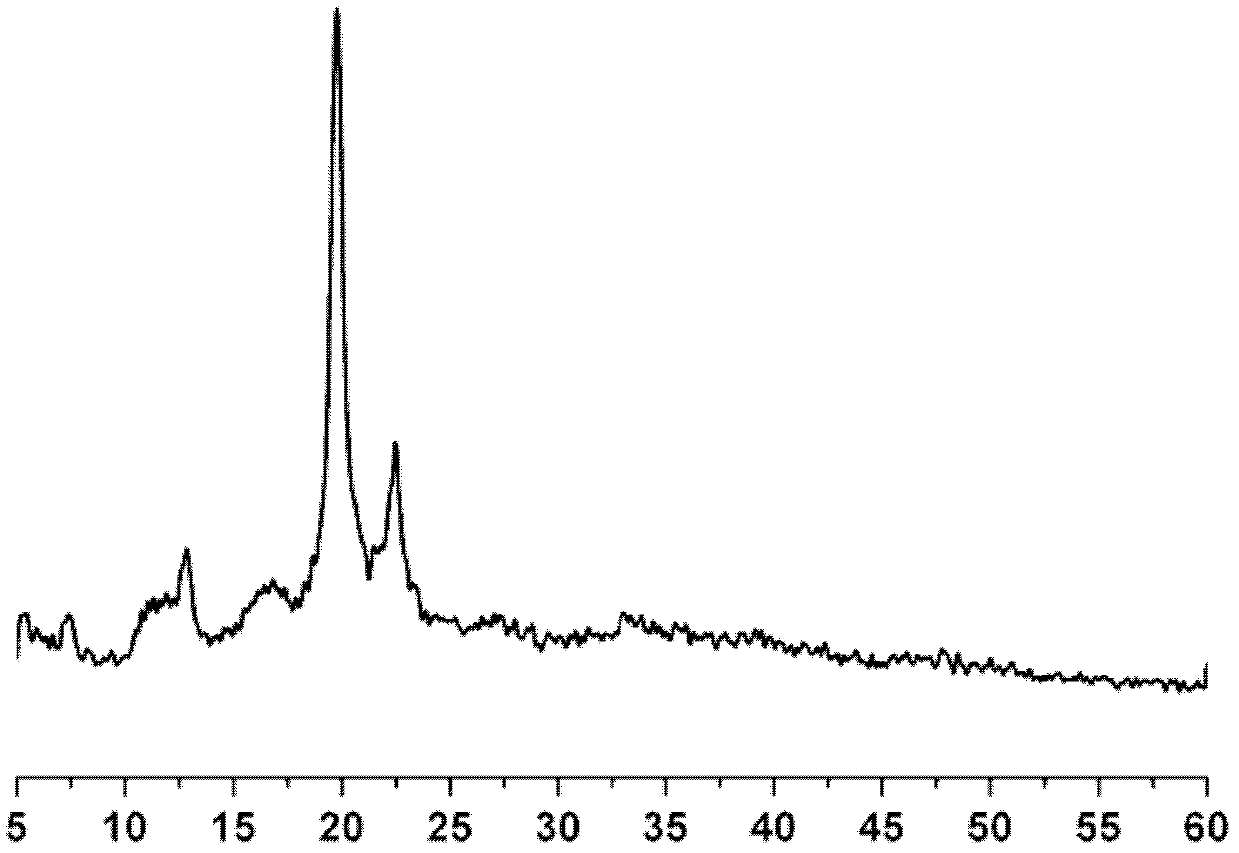
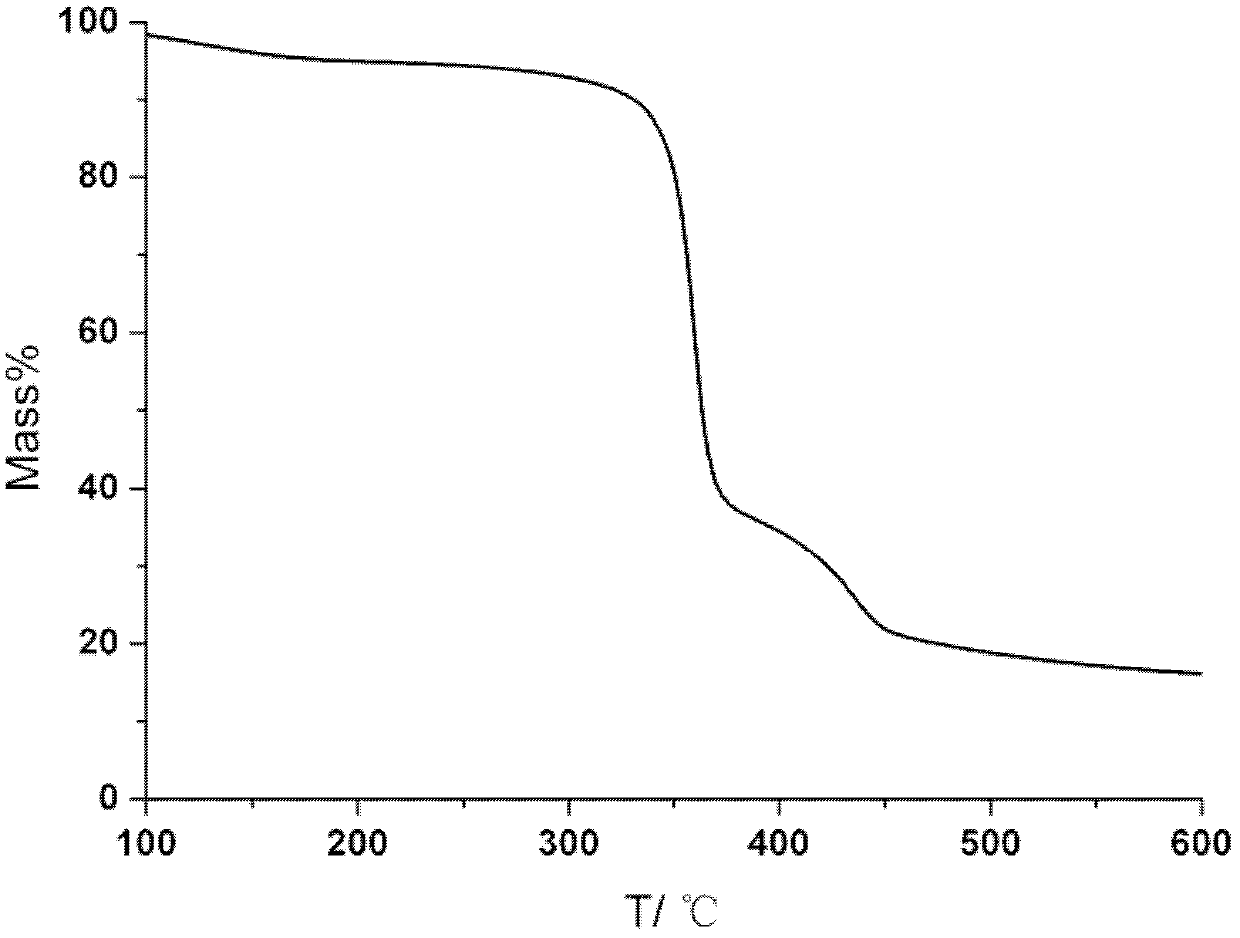
Polyacrylate polymer terminated cyclodextrin polyrotaxane and preparation method thereof
A polyacrylate and cyclodextrin technology, applied in the field of polymer-terminated cyclodextrin polyrotaxane and its preparation, can solve problems such as single method, and achieve the effect of low price and widening scope
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Step a: at room temperature, 5 grams of polyethylene glycol dimercaptoacetate (average molecular weight 6100) aqueous solution with a mass percent concentration of 15% and 42.5 grams of an α-cyclodextrin aqueous solution with a mass percent concentration of 11.27% were stirred Mix evenly, wherein the molar ratio of polyethylene glycol dimercaptoacetate to α-cyclodextrin is 1:40; the mixed solution is left to stand at room temperature for 24 hours to obtain a non-flowing gel-like polypseudorotaxane physical condensation glue;
[0025] Step b: Add 4 grams of dimethylaminoethyl acrylate monomer and 8 mg of azobisisobutyronitrile free radical initiator powder to the polypseudorotaxane physical gel obtained above, stir and mix evenly to obtain a white Heterogeneous suspension; the above-mentioned white heterogeneous suspension was placed in a 365 nm ultraviolet dark box analyzer and reacted for 28 hours;
[0026] Step c: After the reaction is completed, add 400 milliliters ...
Embodiment 2
[0036]Step a: at room temperature, 5 grams of polyethylene glycol dimercapto propionate (average molecular weight 10200) aqueous solution and 50 grams of α-cyclodextrin aqueous solution with a mass percent concentration of 11.27% were stirred under the condition of stirring Mix evenly, wherein the molar ratio of polyethylene glycol dimercaptopropionate to α-cyclodextrin is 1:47; the mixed solution is allowed to stand at room temperature for 24 hours to obtain a non-flowing gel-like polypseudorotaxane physical condensation glue;
[0037] Step b: Add 10 grams of dimethylformamide solution of 30% n-butyl acrylate monomer and 30 mg of azobisisobutyronitrile free radical to the polypseudorotaxane physical gel obtained above Initiator powder, obtain a kind of white heterogeneous suspension after stirring and mixing; Above-mentioned white heterogeneous suspension is placed in 365 nanometer ultraviolet dark box analyzer and reacted for 18 hours;
[0038] Step c: After the reaction is...
Embodiment 3
[0044] Step a: at room temperature, 5 grams of polyethylene glycol dimercaptopropionate (average molecular weight 4200) aqueous solution and 10 grams of α-cyclodextrin aqueous solution with a mass percent concentration of 11.27% were stirred under the condition of stirring Mix evenly, wherein the molar ratio of polyethylene glycol dimercaptopropionate to α-cyclodextrin is 1:9.7; the mixed solution is allowed to stand at room temperature for 24 hours to obtain a non-flowing gel-like polypseudorotaxane physical condensation glue;
[0045] Step b: Add 20 grams of dimethyl methacrylate monomer solution in dimethylformamide with a mass percent concentration of 20% and 80 mg of azobisisobutyronitrile free Base initiator powder, after stirring and mixing, obtain a kind of white heterogeneous suspension; Above-mentioned white heterogeneous suspension is placed in 365 nanometer ultraviolet dark box analyzer and reacted for 24 hours;
[0046] Step c: After the reaction is completed, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com