Blue fluorescent material and preparation method thereof
A technology for blue fluorescent and fluorescent materials, applied in the field of cadmium compound blue fluorescent materials and their preparation, can solve the problems of easy aggregation and crystallization of devices, decreased device life, fluorescence quenching, etc. The effect of good photoelectric activity, good blue light emission performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 60mmol of sulfaquinoxaline, 30mmol of cadmium nitrate, 30mmol of 4,4'-bipyridyl, and 10ml of industrial ethanol into the 25mL polytetrafluoroethylene lining of the stainless steel reactor, and stir to make it evenly mixed.
[0025] Seal the reaction kettle and place it in an oven, heat at 60°C for 24 hours; then cool to room temperature naturally, and open the reaction kettle to obtain yellow blocky crystals.
Embodiment 2
[0027] Add 30mmol of sulfaquinoxaline, 60mmol of cadmium nitrate, 60mmol of 4,4'-bipyridyl, and 15ml of industrial ethanol into the 25mL polytetrafluoroethylene lining of the stainless steel reactor, and stir to make it evenly mixed.
[0028] Seal the reaction kettle and place it in an oven, heat at 100°C for 24h; then cool it down to room temperature naturally, and open the reaction kettle to obtain yellow blocky crystals.
Embodiment 3
[0030] Add 20mmol of sulfaquinoxaline, 80mmol of cadmium nitrate, 80mmol of 4,4'-bipyridine into the 25mL polytetrafluoroethylene lining of the stainless steel reaction kettle, add 15ml of industrial ethanol, and stir to make it evenly mixed.
[0031] Seal the reaction kettle and place it in an oven, heat at 130°C for 24 hours; then cool it down to room temperature naturally, and open the reaction kettle to obtain yellow blocky crystals.
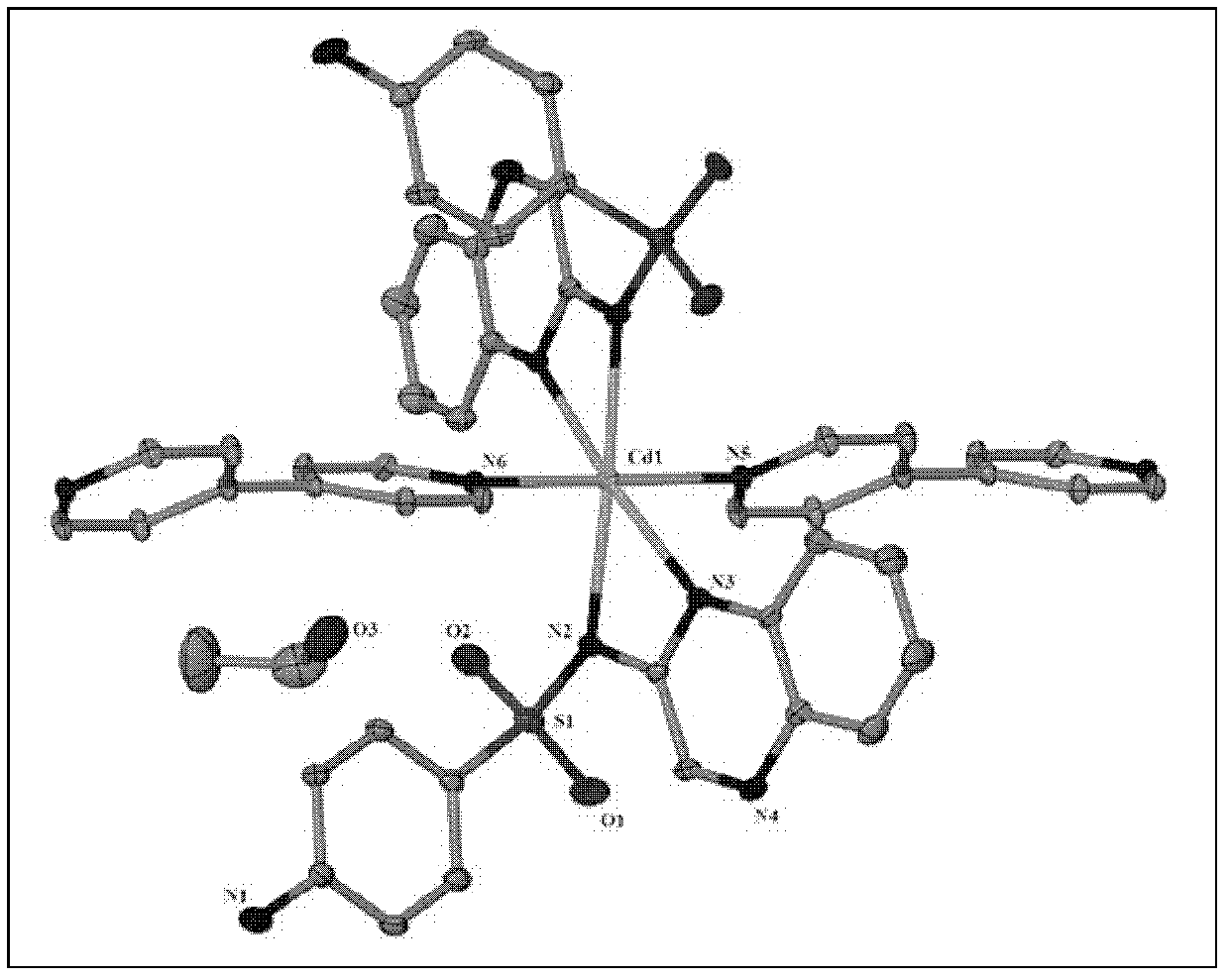
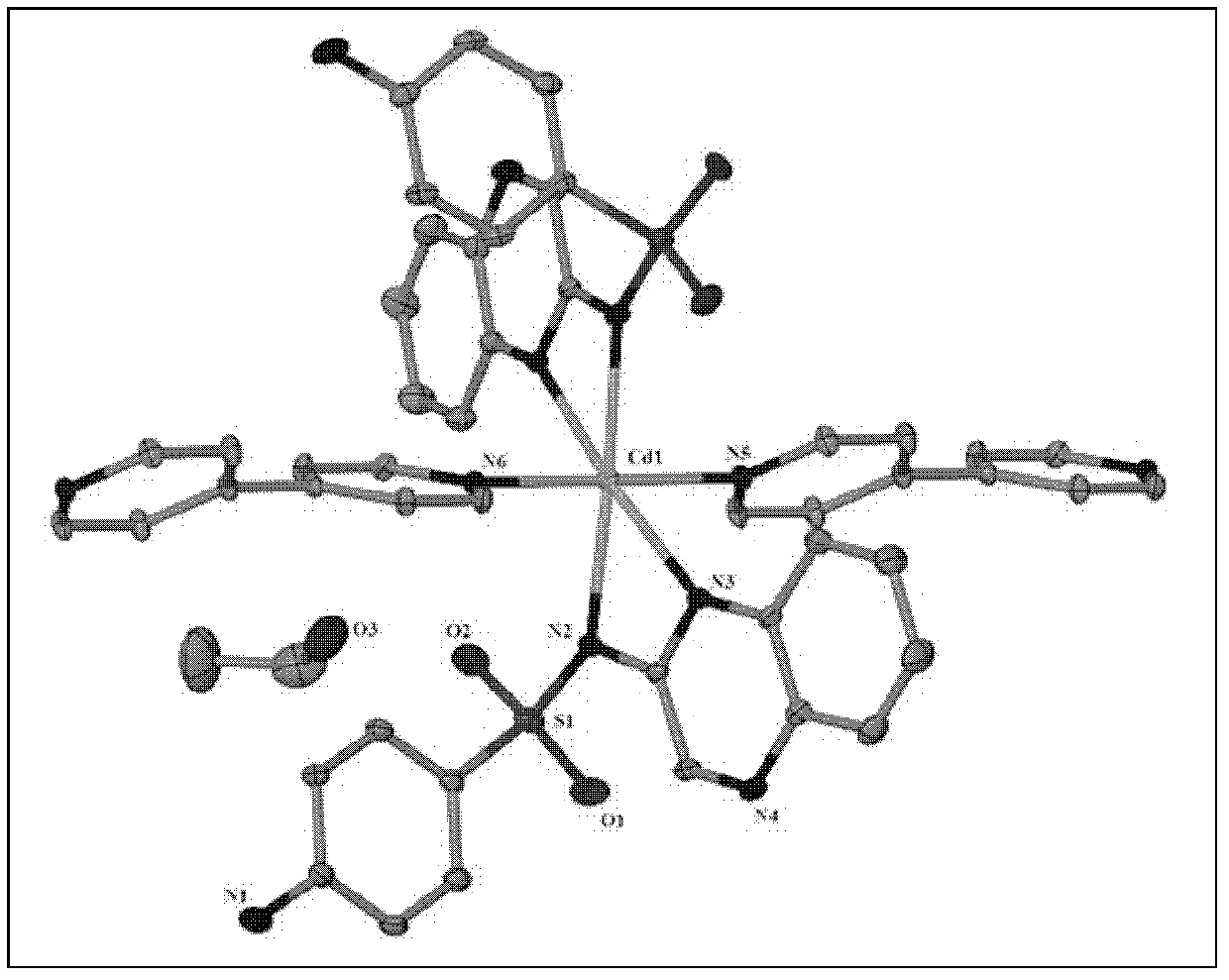
[0032] The crystal is sucked out with a straw and air-dried naturally; after determination, the molecular formula of the cadmium compound is C 42 h 40 CdN 10 o 6 S 2 , the crystal system is monoclinic, the space group is C2 / c, and the unit cell parameters α=90.00°, β=126.005°, γ=90.00°, the cadmium ion is an octahedral coordination mode, 4 nitrogen coordination atoms come from two sulfaquinoxaline ligands, and 2 nitrogen coordination atoms come from two A 4,4'-bipyridine. Its crystal structure is shown in figure 1 shown.
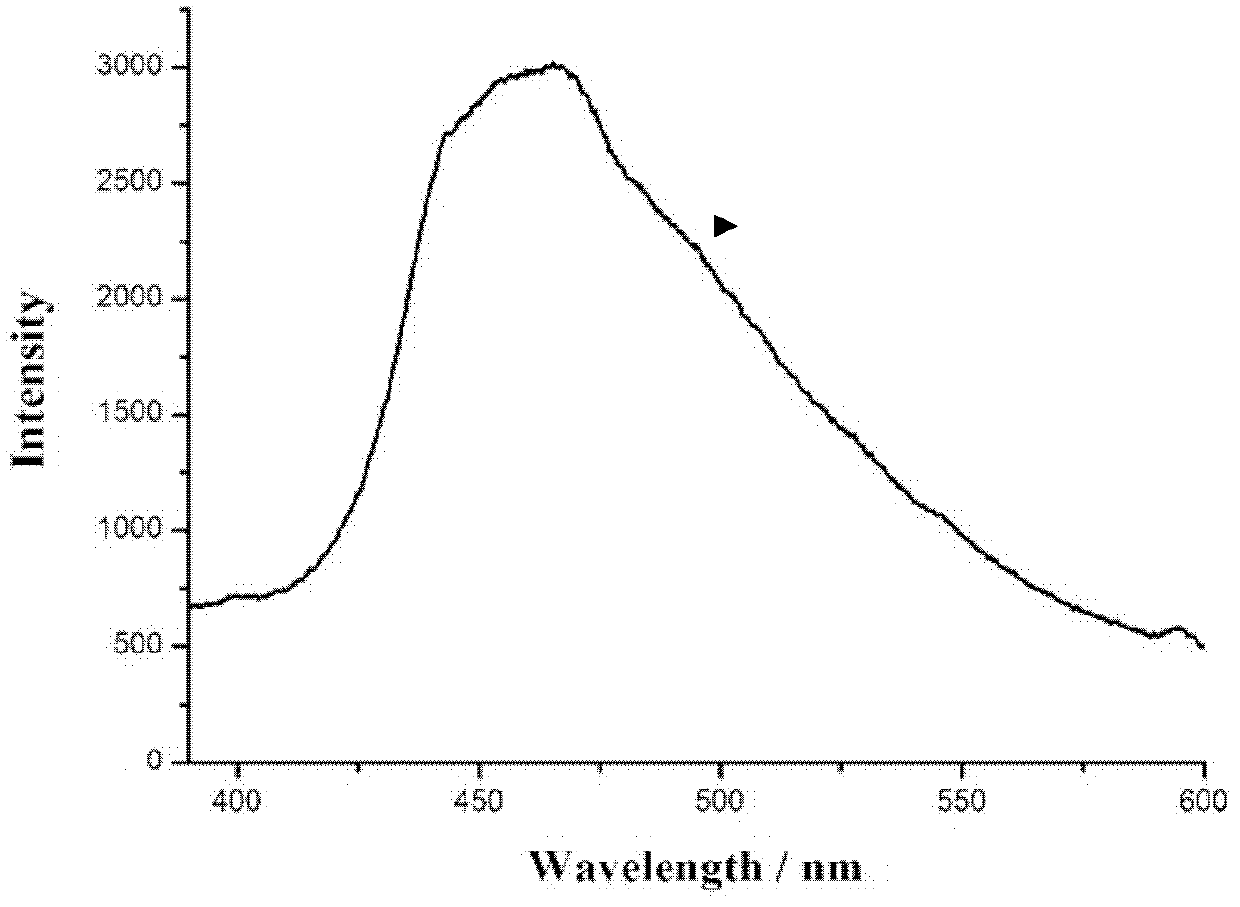
[0033] Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com