Ginsenoside composition for treating cardiovascular and cerebrovascular diseases
A technology of cardiovascular and cerebrovascular diseases and ginsenoside, which is applied in the field of medicine, can solve the problems of difficult determination of content, complex components, low content, etc., and achieve the effect of improving various injuries, exact drug effect, and inhibiting anticoagulant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
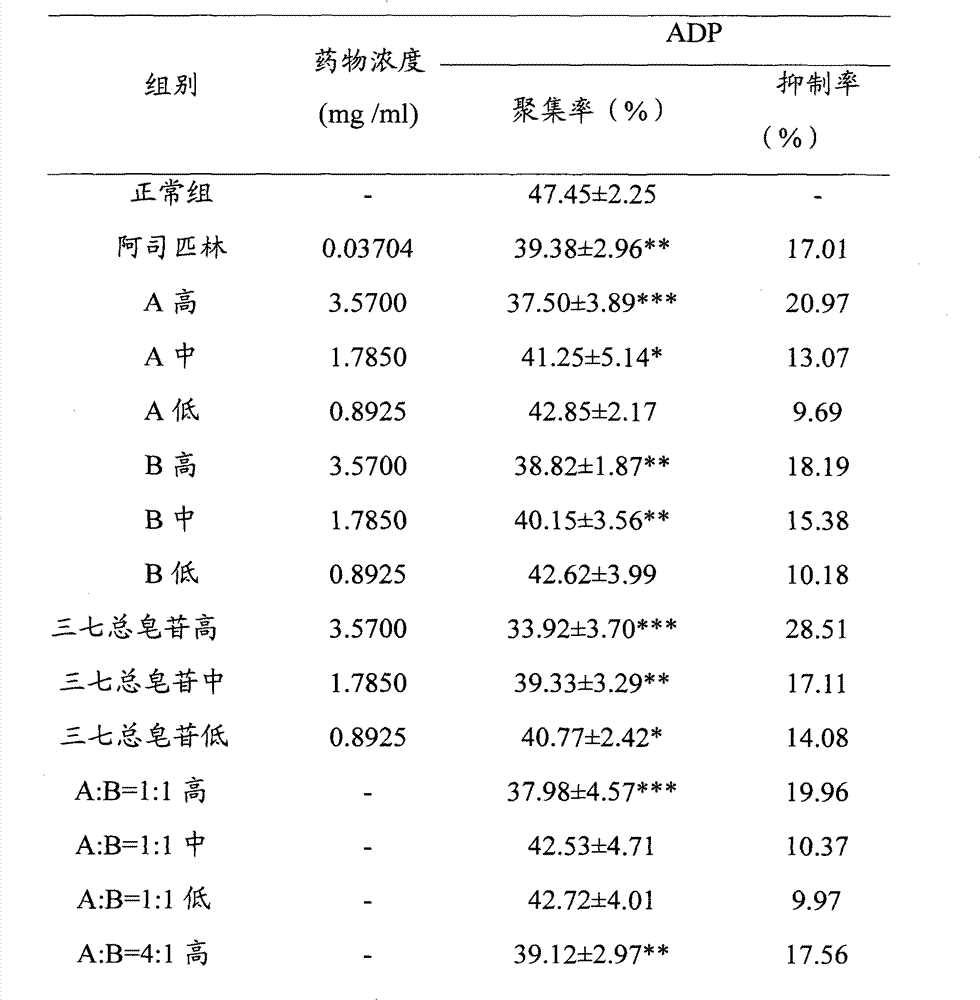
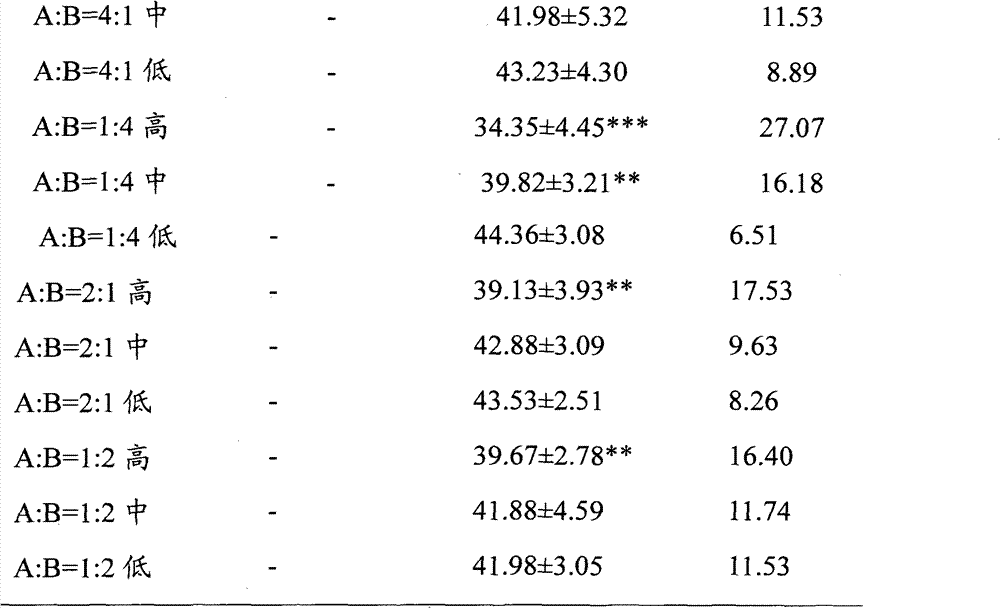
[0028] Embodiment 1, the effect of ginsenoside Rg1, ginsenoside Rb1 and their ratio on ADP-induced platelet aggregation in rabbits in vitro
[0029] 1 drug
[0030] 1.1 Test drug preparation
[0031] Before the experiment, distilled water was used to prepare a solution with a high concentration of 3.5700 mg / ml, a medium concentration of 1.7850 mg / ml, and a low concentration of 0.8925 mg / ml as the final concentration. The different ratio solutions of ginsenoside Rg1 and ginsenoside Rb1 were prepared according to Ginsenoside Rg1 and ginsenoside Rb1 are mixed in different volume proportions of the above-mentioned concentrations. The normal control group used normal saline, wherein 30 μl of Tween was added to every 10 ml of each dose of the test drug and normal saline. The above test drugs were prepared into clear solutions before in vitro administration.
[0032] 1.2 Reagents
[0033]Adenosine diphosphate (ADP): product of Sigma Company; Sodium citrate: Tianjin Chemical Reage...
Embodiment 2
[0064] Example 2 Ginsenoside Rg 1 with Rb 1 Effects of Different Proportions on Specific Myocardial Hypoxia Resistance Model
[0065] 1. Test material:
[0066] Drug: Ginsenoside Rg 1 Ginsenoside Rb 1 Prepare Rg1:Rb1=1:4, 4:1, 1:1 (W / W) mixture respectively; Panax notoginseng saponins, produced by Kunming Pharmaceutical Group Co., Ltd., content 93%, batch number: 20080115; 0.9% chlorinated Sodium injection, produced by Kunming Nanjiang Pharmaceutical Co., Ltd., batch number: 080530h 1 .
[0067] Reagents and equipment: isoproterenol hydrochloride injection (2ml: 1mg Shanghai Hefeng Pharmaceutical Co., Ltd.), 250ml ground-mouth jar, timer, soda lime vaseline, etc.
[0068] Experimental animals: SPF grade ICR mice, ♀ Combined use, W: 18~22g, produced by the laboratory animal laboratory of Kunming Pharmaceutical Group Co., Ltd., production license number: SCXK (Dian) 2009-0001; use license number: SYXK (Dian) 2009-0001.
[0069] 2 Test method:
[0070] The mice were ran...
Embodiment 3
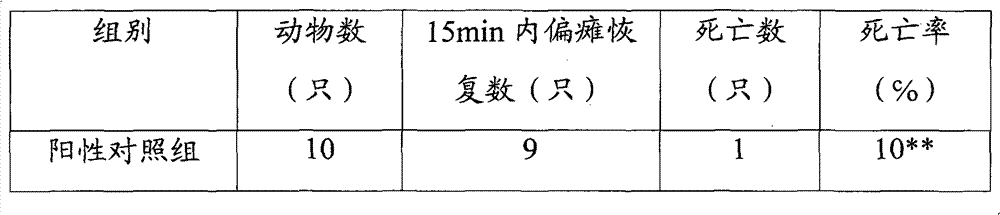
[0078] Example 3: Effects on collagen-epinephrine-induced thrombus formation in mice
[0079] 1 test material
[0080] Drug: Ginsenoside Rg 1 Ginsenoside Rb 1 Prepare Rg1:Rb1=1:4, 4:1, 1:1 (W / W) mixture respectively; Panax notoginseng saponins, produced by Kunming Pharmaceutical Group Co., Ltd., content 93%, batch number: 20080115; 0.9% chlorinated Sodium injection, produced by Kunming Nanjiang Pharmaceutical Co., Ltd., batch number: 080530h 1 . Bayer Aspirin is produced by Bayer Corporation of the United States.
[0081] Reagents: collagen, epinephrine, etc.
[0082] Experimental animals: SPF grade ICR mice, ♀ Combined use, W: 18~22g, produced by the laboratory animal laboratory of Kunming Pharmaceutical Group Co., Ltd., production license number: SCXK (Dian) 2009-0001; use license number: SYXK (Dian) 2009-0001.
[0083]2 Test method:
[0084] 60 mice were randomly divided into 8 groups: Rg1:Rb1 (5:1) group, Rg1:Rb1 (4:1) group, Rg1:Rb1 (1:1), Rg1:Rb1 (1:4), Rg1: Rb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com