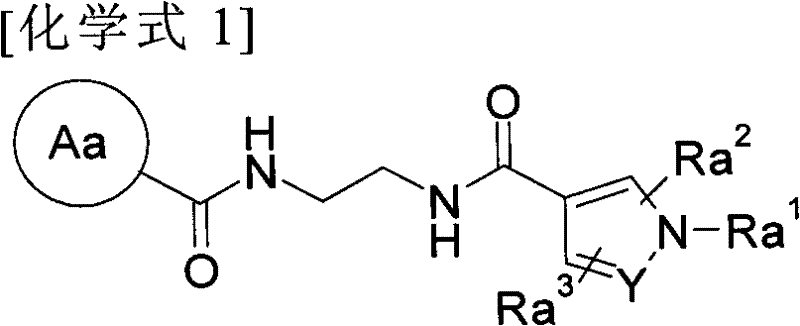
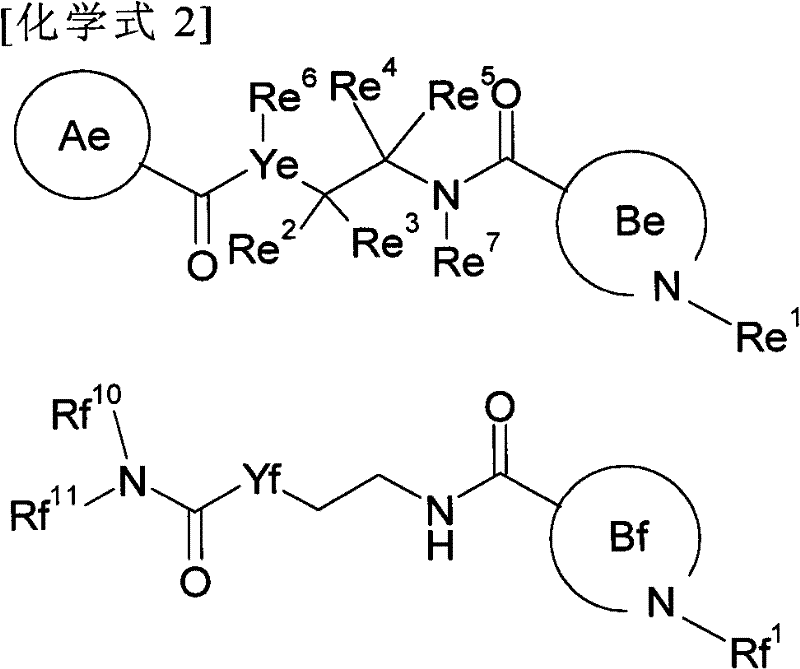
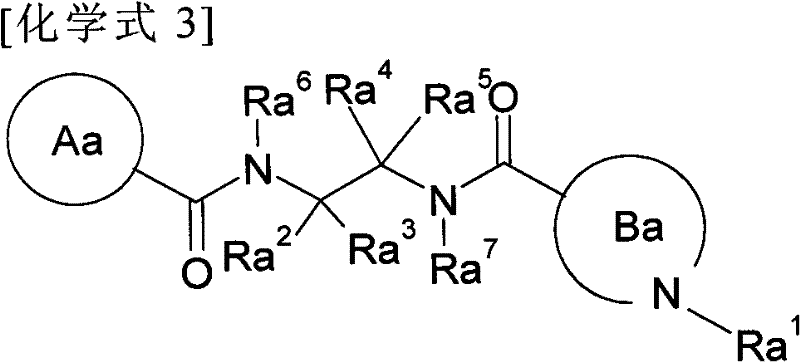
Diacylethylenediamine compound
A compound, carbamoyl technology, applied in the field of diacylethylenediamine compounds, can solve problems such as no disclosure or implied compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0124] This preparation method is a step of converting the carboxyl group of the compound (I-a) prepared by the first preparation method as the compound of the present invention into a formamide group or a bioisostere of the carboxyl group.
[0125] For example, in Z 1 for-CO-NH-SO 2 -C 1-6 Alkyl or -CO-NH-SO 2 -N(C 1-6 alkyl) 2 In the case of , it can be prepared by a fusion reaction with the corresponding sulfonamide; for example, in Z 1 for-CONH 2 In the case of , it can be prepared by a fusion reaction using ammonium chloride, ammonium hydroxide, ammonia solution, etc.; for example, in Z 1 In the case of a tetrazolyl group, after being induced into formamide, it is dehydrated to induce a nitro group by a conventional method, and then reacted with sodium azide to produce it. Alternatively, the carboxyl group can be converted to its bioisostere using methods known to those skilled in the art.
[0126] (Raw material synthesis 1)
[0127]
[0128] (where, A 1 Repr...
Embodiment
[0190] The preparation method of the compound represented by formula (I) or its salt will be described in more detail below based on the examples. Furthermore, the present invention is not limited to the compounds described in the following examples. In addition, the production methods of the raw material compounds are shown in the production examples. In addition, the preparation method of the compound represented by formula (I) is not limited to the preparation method of the specific examples shown below, and the compound represented by formula (I) can be prepared by a combination of these preparation methods or methods known to those skilled in the art. shows the compound.
[0191] In addition, in Examples, Preparation Examples, and the following tables, the following abbreviations are sometimes used.
[0192] Pr: Preparation number (preparation compound with " / Cl" behind the preparation number means that the preparation compound is isolated in the form of hydrochloride, ...
preparation example 1
[0194] Under ice-cooling, ethyl trans-4-hydroxycyclohexanecarboxylate (8g), benzyl 4-hydroxybenzoate (11.69g), 1,1'-(azobiscarbonyl)dipiperidine ( 15.24 g), THF (150 ml) was added dropwise to a mixture of tributylphosphine (14.9 ml), stirred at room temperature for 2 hours, and then stirred at 60° C. for 10 hours. After returning the reaction mixture to room temperature, it was filtered, washed with THF, and the filtrate was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (hexane:ethyl acetate=85:15) to give benzyl 4-{[cis-4-(ethoxycarbonyl)cyclohexyl]oxy}benzoate 8.55 g of colorless oily substance of base ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com