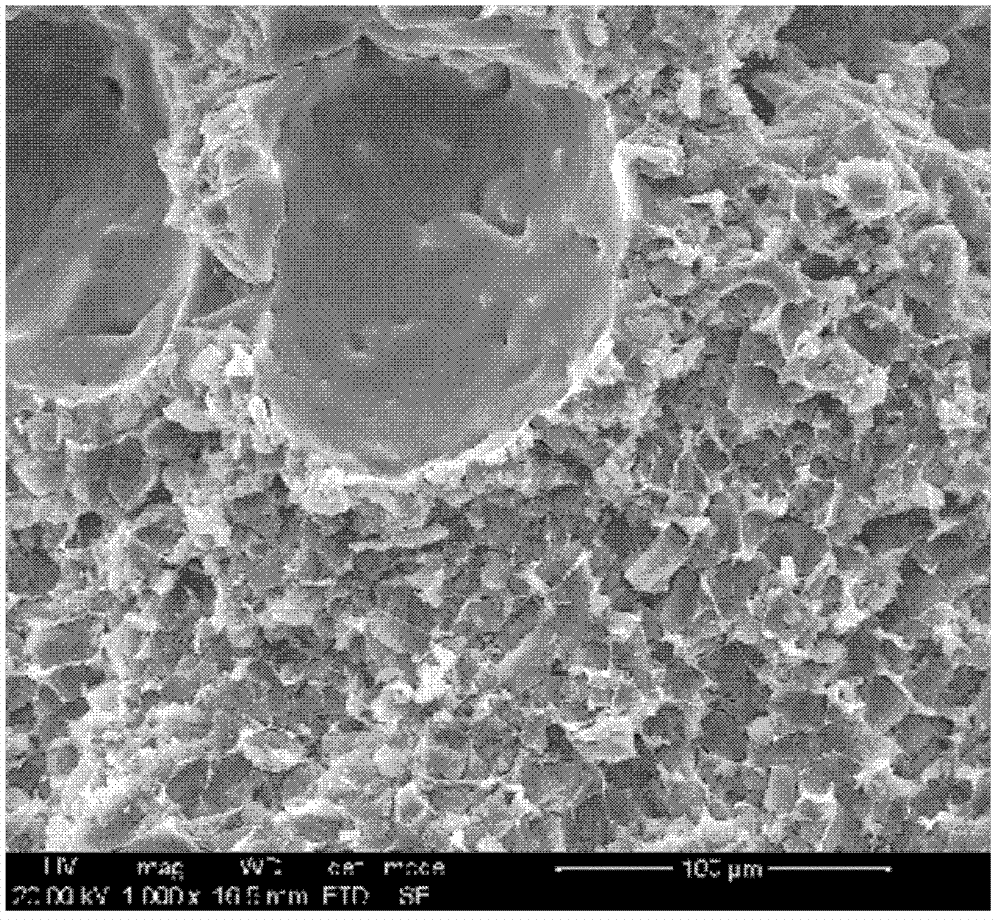
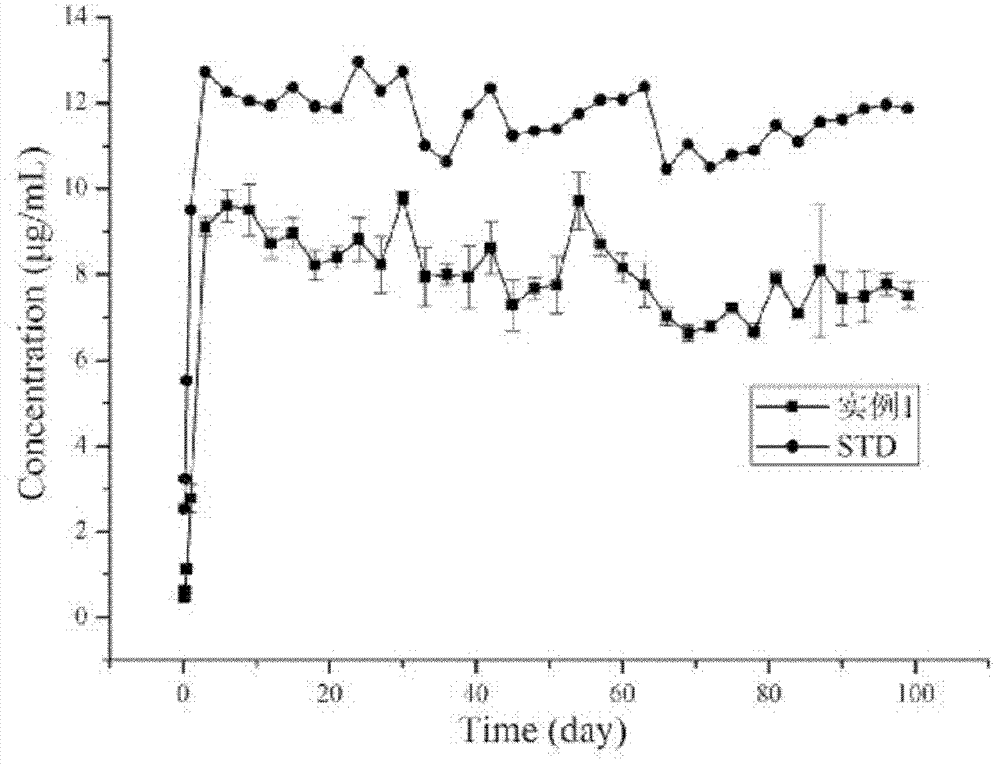
Medicinal slow release inserted slice possessing layer-by-layer assembly mosaic structure
A layer-by-layer assembly and mosaic technology, which is applied in medical science, making drugs into special physical or ingestible devices, pill delivery, etc., can solve the problems of short validity period, hard material, complicated process, etc., to achieve simple operation, The effect of low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Prepare the mixed solution of polycaprolactone / triamcinolone acetonide:
[0048] First, dissolve polycaprolactone with a number average molecular weight of 20,000 in dichloromethane to prepare a solution a with a solid phase concentration of 10 mg / ml; then dissolve triamcinolone acetonide in acetone to prepare a drug solution with a concentration of 5 mg / ml b; according to the requirements of the drug loading, take two solutions of appropriate volumes and mix them, and after half an hour of closed ultrasonication, stand-by;
[0049] (2) Spraying:
[0050] Using spraying equipment, the pressure is selected as 0.15MPa, and the mixed solution is sprayed onto the collecting plate that has been heated to 40°C in advance;
[0051] (3) Introduce hot air:
[0052] After 20 seconds of spraying each time, pass hot air at 40°C over the collecting plate for 15 seconds; repeat this process until the plant reaches the actual required thickness;
[0053] (4) Drying:
[0054] P...
Embodiment 2
[0060] (1) Prepare a mixed solution of polycaprolactone / tacrolimus:
[0061] First, dissolve polycaprolactone with a number average molecular weight of 20,000 in dichloromethane to prepare a solution a with a solid phase concentration of 75 mg / ml; then dissolve tacrolimus in acetone to prepare a drug with a concentration of 3 mg / ml Solution b; according to the requirements of the drug loading, take two solutions of appropriate volumes and mix them, and after half an hour of closed ultrasonication, stand-by;
[0062] (2) Spraying:
[0063] Using spraying equipment, the pressure is selected as 0.3MPa, and the mixed solution is sprayed onto the collecting plate that has been heated to 60°C in advance;
[0064] (3) Introduce hot air:
[0065] After 40 seconds of spraying each time, pass hot air at 60°C over the collecting plate for 45 seconds; repeat this process until the plant reaches the actual required thickness;
[0066] (4) Drying:
[0067] The sample is placed in a vacu...
Embodiment 3
[0071] (1) Prepare the mixed solution of polycaprolactone / triamcinolone acetonide:
[0072] First, dissolve polycaprolactone with a number average molecular weight of 80,000 in dichloromethane to prepare a solution a with a solid phase concentration of 10 mg / ml; then dissolve triamcinolone acetonide in acetone to prepare a drug solution with a concentration of 7 mg / ml b; according to the requirements of the drug loading, take two solutions of appropriate volumes and mix them, and after half an hour of closed ultrasonication, stand-by;
[0073] (2) Spraying:
[0074] Using spraying equipment, the pressure is selected as 0.15MPa, and the mixed solution is sprayed onto the collecting plate that has been heated to 40°C in advance;
[0075] (3) Introduce hot air:
[0076] After 20 seconds of spraying each time, pass hot air at 40°C over the collecting plate for 15 seconds; repeat this process until the plant reaches the actual required thickness;
[0077] (4) Drying:
[0078] P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com