Ss-galactosidase derived from bacillus circulans
A technology of Bacillus circulans and galactosidase, applied in the direction of glycosylase, enzyme, bacteria, etc., can solve the problem of unclear coding activity of protein and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
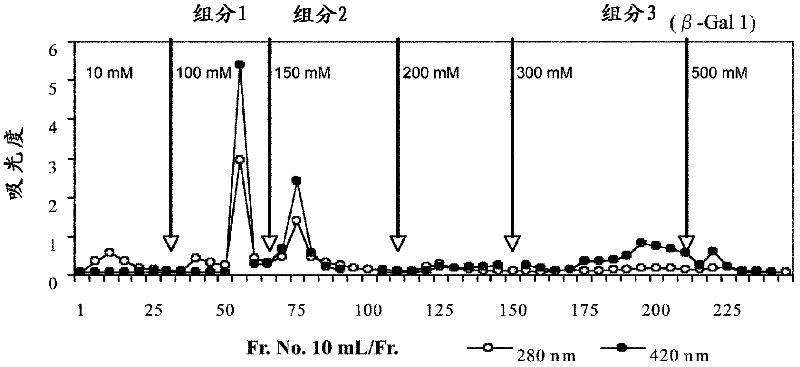
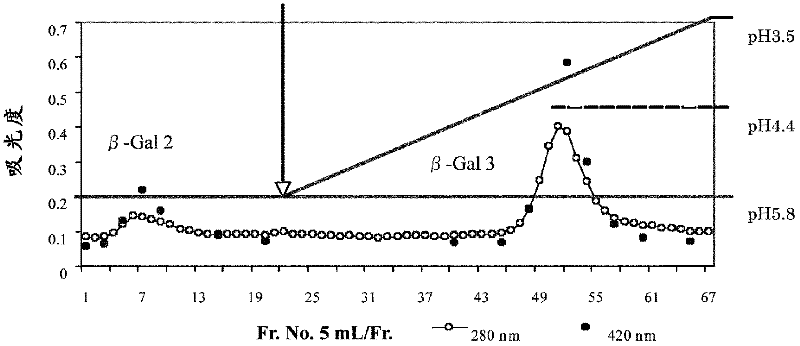
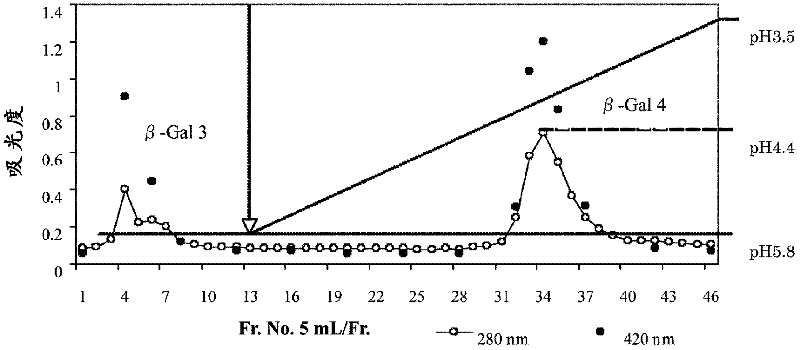
[0089] 1. Purification of β-galactosidase from Bacillus circulans
[0090] (a) β-galactosidase activity assay
[0091] In the following purifications, β-galactosidase activity was measured by method (i) using 2-nitrophenyl β-D-galactopyranoside (ONPG) as a substrate and method (i) using lactose as a substrate Method (ii) These two types are measured. All were carried out according to the method described in Non-Patent Document 1. In addition, the protein concentration is represented by the absorbance at 280 nm.
[0092] i) ONPG method
[0093] 1.98 ml of 100 mM phosphate buffer (pH 6.0) containing 0.245% ONPG was preheated at 40° C. for 10 minutes. 20 μl of the sample was added thereto, and after reacting at 40° C. for 10 minutes, 2.0 ml of a 10% sodium carbonate solution was added to stop the reaction. The absorbance at 420 nm of the reaction solution was measured, and the activity when 1 μmol of 2-nitrophenol was produced per minute was regarded as 1 U to calculate the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com